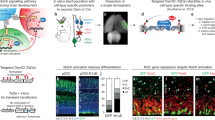
Key Points
-
Notch is an evolutionarily conserved, membrane-bound receptor whose ligands are also membrane-bound. As ligand-mediated enzymatic cleavage of Notch results in nuclear signalling through canonical and non-canonical pathways, Notch is considered a key integrator of environmental signals and can be thought of as a 'membrane-bound transcription factor'.
-
The Notch signalling cascade is well known for its role during CNS development. However, technical advancements in gene and protein manipulation strategies have revealed both conserved and novel roles for Notch signalling in the adult brain in processes as diverse as neural stem cell maintenance, the regulation of neuronal plasticity and even survival.
-
In neural stem cells, Notch regulates the cell cycle to balance stem cell maintenance with production of daughter cells. The ability of Notch to regulate proliferation is highly cell-type dependent, with more neuronally committed progeny responsive to a variety of environmental cues, not just Notch ligands.
-
Notch signalling also regulates neuronal migration, in part through reelin–DAB (disabled homologue) signalling. Additional research suggests that Notch-mediated regulation of migration may also rely on indirect regulation of microtubule stability.
-
Neuronal dendritic plasticity in the adult brain is reliant on Notch signalling, but is notably age- and 'cell stage'-dependent. For example, Notch signalling regulates dendritic arborization in nascent neurons, but may only fine-tune dendritic spines in 'older' neurons.
-
Notch signalling regulates synaptic plasticity and behaviour, but the direction of this regulation is exquisitely dose- and context-dependent.
-
In healthy, non-aged animals, the regulation of neuron survival by Notch signalling is difficult to separate from its effects on dendritic arborization and spine maintenance. However, in models of neurodegenerative disease, Notch signalling seems to drive dendritic atrophy and may lead to neuron death.
-
Increasing evidence indicates that activity-induced Notch signalling in neurons has an important role in cellular forms of memory and behavioural demonstration of memory.
-
Disruptions in Notch signalling result in diseases that have a strong neurodegenerative component. Therefore, comprehension of Notch signalling in the adult — and fully understanding how it is similar to or different from that in the developing animal — has substantial therapeutic implications.
-
Despite advances in our knowledge of the influence of Notch signalling on a wide range of cell types, stages and functions, it remains challenging to predict the outcome of activation. It is crucial to consider dose-, context- and age-dependence, and to consider the crosstalk with other signalling pathways (which are likely to be as dynamic as the Notch pathway).
Abstract
The Notch pathway is often regarded as a developmental pathway, but components of Notch signalling are expressed and active in the adult brain. With the advent of more sophisticated genetic manipulations, evidence has emerged that suggests both conserved and novel roles for Notch signalling in the adult brain. Not surprisingly, Notch is a key regulator of adult neural stem cells, but it is increasingly clear that Notch signalling also has roles in the regulation of migration, morphology, synaptic plasticity and survival of immature and mature neurons. Understanding the many functions of Notch signalling in the adult brain, and its dysfunction in neurodegenerative disease and malignancy, is crucial to the development of new therapeutics that are centred around this pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Louvi, A. & Artavanis-Tsakonas, S. Notch signalling in vertebrate neural development. Nature Rev. Neurosci. 7, 93–102 (2006).
Yoon, K. & Gaiano, N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nature Neurosci. 8, 709–715 (2005).
Berezovska, O., Xia, M. Q. & Hyman, B. T. Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J. Neuropathol. Exp. Neurol. 57, 738–745 (1998).
Sestan, N., Artavanis-Tsakonas, S. & Rakic, P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science 286, 741–746 (1999). This paper is the first to show Notch function in postmitotic neurons in regulating density-dependent dendritic arborization.
Li, L. et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nature Genet. 16, 243–251 (1997).
Oda, T. et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nature Genet. 16, 235–242 (1997).
Joutel, A. et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383, 707–710 (1996).
Isidor, B. et al. Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nature Genet. 6 Mar 2011 (doi:10.1038/ng.778).
Simpson, M. A. et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nature Genet. 6 Mar 2011 (doi:10.1038/ng.779).
Fischer, D. F. et al. Activation of the Notch pathway in Down syndrome: cross-talk of Notch and APP. FASEB J. 19, 1451–1458 (2005).
Veeraraghavalu, K., Choi, S. H., Zhang, X. & Sisodia, S. S. Presenilin 1 mutants impair the self-renewal and differentiation of adult murine subventricular zone-neuronal progenitors via cell-autonomous mechanisms involving notch signaling. J. Neurosci. 30, 6903–6915 (2010).
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Kopan, R. & Ilagan, M. X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 (2009).
D'Souza, B., Meloty-Kapella, L. & Weinmaster, G. Canonical and non-canonical Notch ligands. Curr. Top. Dev. Biol. 92, 73–129 (2010).
Kovall, R. A. & Blacklow, S. C. Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr. Top. Dev. Biol. 92, 31–71 (2010).
Yamamoto, S., Charng, W. L. & Bellen, H. J. Endocytosis and intracellular trafficking of Notch and its ligands. Curr. Top. Dev. Biol. 92, 165–200 (2010).
Bray, S. & Bernard, F. Notch targets and their regulation. Curr. Top. Dev. Biol. 92, 253–275 (2010).
Sanalkumar, R., Dhanesh, S. B. & James, J. Non-canonical activation of Notch signaling/target genes in vertebrates. Cell. Mol. Life Sci. 67, 2957–2968 (2010).
De Strooper, B. & Annaert, W. Novel research horizons for presenilins and γ-secretases in cell biology and disease. Annu. Rev. Cell Dev. Biol. 26, 235–260 (2010).
Yang, M. S. et al. Among γ-secretase substrates Notch1 alone is sufficient to block neurogenesis but does not confer self-renewal properties to neural stem cells. Biochem. Biophys. Res. Commun. 404, 133–138 (2011).
Extance, A. Alzheimer's failure raises questions about disease-modifying strategies. Nature Rev. Drug Discov. 9, 749–751 (2010).
Alvarez-Buylla, A. & Lim, D. A. For the long run: maintaining germinal niches in the adult brain. Neuron 41, 683–686 (2004).
Andreu-Agullo, C., Morante-Redolat, J. M., Delgado, A. C. & Farinas, I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nature Neurosci. 12, 1514–1523 (2009).
Breunig, J. J., Silbereis, J., Vaccarino, F. M., Sestan, N. & Rakic, P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc. Natl Acad. Sci. USA 104, 20558–20563 (2007).
Givogri, M. I. et al. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev. Neurosci. 28, 81–91 (2006).
Stump, G. et al. Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech. Dev. 114, 153–159 (2002).
Ostenfeld, T. & Svendsen, C. N. Requirement for neurogenesis to proceed through the division of neuronal progenitors following differentiation of epidermal growth factor and fibroblast growth factor-2-responsive human neural stem cells. Stem Cells 22, 798–811 (2004).
Rakic, P., Ayoub, A. E., Breunig, J. J. & Dominguez, M. H. Decision by division: making cortical maps. Trends Neurosci. 32, 291–301 (2009).
Chapouton, P. et al. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J. Neurosci. 30, 7961–7974 (2010).
Ables, J. L. et al. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J. Neurosci. 30, 10484–10492 (2010).
Ehm, O. et al. RBPJκ-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J. Neurosci. 30, 13794–13807 (2010).
Imayoshi, I., Sakamoto, M., Yamaguchi, M., Mori, K. & Kageyama, R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 30, 3489–3498 (2010).
Borghese, L. et al. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells 28, 955–964 (2010).
Guentchev, M. & McKay, R. D. Notch controls proliferation and differentiation of stem cells in a dose-dependent manner. Eur. J. Neurosci. 23, 2289–2296 (2006).
Hashimoto-Torii, K. et al. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 60, 273–284 (2008).
Sibbe, M., Forster, E., Basak, O., Taylor, V. & Frotscher, M. Reelin and Notch1 cooperate in the development of the dentate gyrus. J. Neurosci. 29, 8578–8585 (2009).
Brandt, M. D., Maass, A., Kempermann, G. & Storch, A. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neurogenesis. Eur. J. Neurosci. 32, 1256–1264 (2010).
Crews, L. et al. α-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J. Neurosci. 28, 4250–4260 (2008).
Simon-Areces, J., Membrive, G., Garcia-Fernandez, C., Garcia-Segura, L. M. & Arevalo, M. A. Neurogenin 3 cellular and subcellular localization in the developing and adult hippocampus. J. Comp. Neurol. 518, 1814–1824 (2010).
Ferrari-Toninelli, G., Bonini, S. A., Bettinsoli, P., Uberti, D. & Memo, M. Microtubule stabilizing effect of notch activation in primary cortical neurons. Neuroscience 154, 946–952 (2008).
Giniger, E. A role for Abl in Notch signaling. Neuron 20, 667–681 (1998).
Crowner, D., Le Gall, M., Gates, M. A. & Giniger, E. Notch steers Drosophila ISNb motor axons by regulating the Abl signaling pathway. Curr. Biol. 13, 967–972 (2003).
Le Gall, M., De Mattei, C. & Giniger, E. Molecular separation of two signaling pathways for the receptor, Notch. Dev. Biol. 313, 556–567 (2008).
Hassan, B. A. et al. atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron 25, 549–561 (2000).
Berezovska, O. et al. Notch1 inhibits neurite outgrowth in postmitotic primary neurons. Neuroscience 93, 433–439 (1999).
Redmond, L., Oh, S. R., Hicks, C., Weinmaster, G. & Ghosh, A. Nuclear Notch1 signaling and the regulation of dendritic development. Nature Neurosci. 3, 30–40 (2000).
Salama-Cohen, P., Arevalo, M. A., Grantyn, R. & Rodriguez-Tebar, A. Notch and NGF/p75NTR control dendrite morphology and the balance of excitatory/inhibitory synaptic input to hippocampal neurones through Neurogenin 3. J. Neurochem. 97, 1269–1278 (2006).
Huang, E. J. et al. Targeted deletion of numb and numblike in sensory neurons reveals their essential functions in axon arborization. Genes Dev. 19, 138–151 (2005).
Santolini, E. et al. Numb is an endocytic protein. J. Cell Biol. 151, 1345–1352 (2000).
Zhao, C., Deng, W. & Gage, F. H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 (2008).
Dahlhaus, M. et al. Notch1 signaling in pyramidal neurons regulates synaptic connectivity and experience-dependent modifications of acuity in the visual cortex. J. Neurosci. 28, 10794–10802 (2008). This is one of the first studies to show activity-dependent Notch signalling in vivo . Using genetic approaches the authors show that Notch regulates structural and functional plasticity of pyramidal neurons.
Costa, R. M., Honjo, T. & Silva, A. J. Learning and memory deficits in Notch mutant mice. Curr. Biol. 13, 1348–1354 (2003).
Givogri, M. I. et al. Central nervous system myelination in mice with deficient expression of Notch1 receptor. J. Neurosci. Res. 67, 309–320 (2002).
Heitzler, P. & Simpson, P. The choice of cell fate in the epidermis of Drosophila. Cell 64, 1083–1092 (1991).
Presente, A., Boyles, R. S., Serway, C. N., de Belle, J. S. & Andres, A. J. Notch is required for long-term memory in Drosophila. Proc. Natl Acad. Sci. USA 101, 1764–1768 (2004).
Ge, X. et al. Notch signaling in Drosophila long-term memory formation. Proc. Natl Acad. Sci. USA 101, 10172–10176 (2004).
Pavlopoulos, E., Anezaki, M. & Skoulakis, E. M. Neuralized is expressed in the α/β lobes of adult Drosophila mushroom bodies and facilitates olfactory long-term memory formation. Proc. Natl Acad. Sci. USA 105, 14674–14679 (2008).
Wang, Y. et al. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc. Natl Acad. Sci. USA 101, 9458–9462 (2004).
Conboy, L. et al. Notch signalling becomes transiently attenuated during long-term memory consolidation in adult Wistar rats. Neurobiol. Learn. Mem. 88, 342–351 (2007). This study carefully examines the time course of Notch signalling during memory formation. Furthermore, it identifies a novel NICD fragment.
Tagami, S. et al. Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1. Mol. Cell. Biol. 28, 165–176 (2008).
de Bivort, B. L., Guo, H. F. & Zhong, Y. Notch signaling is required for activity-dependent synaptic plasticity at the Drosophila neuromuscular junction. J. Neurogenet. 23, 395–404 (2009).
Alberi, L. et al. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron 69, 437–444 (2011). This is the first study to demonstrate that Notch is present at the rodent synapse. They also elegantly show that Notch signalling is dependent on neuronal activity and regulates the structure and function of neurons in vivo.
Lieber, T., Kidd, S. & Struhl, G. DSL-Notch signaling in the Drosophila brain in response to olfactory stimulation. Neuron 69, 468–481 (2011). Using the power of Drosophila genetics, this study illuminates the dynamics of activity-related Notch signalling elicited by specific odorants.
McGeer, P. L., Kawamata, T. & McGeer, E. G. Localization and possible functions of presenilins in brain. Rev. Neurosci. 9, 1–15 (1998).
Franberg, J., Karlstrom, H., Winblad, B., Tjernberg, L. O. & Frykman, S. γ-Secretase dependent production of intracellular domains is reduced in adult compared to embryonic rat brain membranes. PLoS ONE 5, e9772 (2010).
Placanica, L., Zhu, L. & Li, Y. M. Gender- and age-dependent γ-secretase activity in mouse brain and its implication in sporadic Alzheimer disease. PLoS ONE 4, e5088 (2009).
Song, W. et al. Proteolytic release and nuclear translocation of Notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc. Natl Acad. Sci. USA 96, 6959–6963 (1999).
Chen, C. D., Oh, S. Y., Hinman, J. D. & Abraham, C. R. Visualization of APP dimerization and APP-Notch2 heterodimerization in living cells using bimolecular fluorescence complementation. J. Neurochem. 97, 30–43 (2006).
Fassa, A., Mehta, P. & Efthimiopoulos, S. Notch 1 interacts with the amyloid precursor protein in a Numb-independent manner. J. Neurosci. Res. 82, 214–224 (2005).
Oh, S. Y., Chen, C. D. & Abraham, C. R. Cell-type dependent modulation of Notch signaling by the amyloid precursor protein. J. Neurochem. 113, 262–274 (2010).
Oh, S. Y. et al. Amyloid precursor protein interacts with notch receptors. J. Neurosci. Res. 82, 32–42 (2005).
Roncarati, R. et al. The γ-secretase-generated intracellular domain of β-amyloid precursor protein binds Numb and inhibits Notch signaling. Proc. Natl Acad. Sci. USA 99, 7102–7107 (2002).
Kyriazis, G. A. et al. Numb endocytic adapter proteins regulate the transport and processing of the amyloid precursor protein in an isoform-dependent manner: implications for Alzheimer disease pathogenesis. J. Biol. Chem. 283, 25492–25502 (2008).
Kyriazis, G. A. et al. Stress-induced switch in Numb isoforms enhances Notch-dependent expression of subtype-specific transient receptor potential channel. J. Biol. Chem. 285, 6811–6825 (2010).
Ishikura, N. et al. Notch-1 activation and dendritic atrophy in prion disease. Proc. Natl Acad. Sci. USA 102, 886–891 (2005).
Spilman, P. et al. A γ-secretase inhibitor and quinacrine reduce prions and prevent dendritic degeneration in murine brains. Proc. Natl Acad. Sci. USA 105, 10595–10600 (2008).
Ferron, S. R. et al. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J. Neurosci. 29, 14394–14407 (2009). This study details the fascinating finding that age-related telomere changes may fundamentally alter neural stem cell and daughter cell behavior through p53 and Notch.
Oya, S. et al. Attenuation of Notch signaling promotes the differentiation of neural progenitors into neurons in the hippocampal CA1 region after ischemic injury. Neuroscience 158, 683–692 (2009).
Wang, L. et al. The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience 158, 1356–1363 (2009).
Wang, X., Mao, X., Xie, L., Greenberg, D. A. & Jin, K. Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J. Cereb. Blood Flow Metab. 29, 1644–1654 (2009).
Androutsellis-Theotokis, A. et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442, 823–826 (2006).
Alberi, L. et al. Neonatal stroke in mice causes long-term changes in neuronal Notch-2 expression that may contribute to prolonged injury. Stroke 41, S64–S71 (2010).
Arumugam, T. V. et al. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nature Med. 12, 621–623 (2006).
Hellstrom, M. et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 (2007).
John, G. R. et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nature Med. 8, 1115–1121 (2002).
Wang, S. et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63–75 (1998).
Magnus, T. et al. Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes. J. Neurosci. Res. 85, 2126–2137 (2007).
Morga, E. et al. Jagged1 regulates the activation of astrocytes via modulation of NFκB and JAK/STAT/SOCS pathways. Glia 57, 1741–1753 (2009).
Sofroniew, M. V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 (2009).
Chen, J., Leong, S. Y. & Schachner, M. Differential expression of cell fate determinants in neurons and glial cells of adult mouse spinal cord after compression injury. Eur. J. Neurosci. 22, 1895–1906 (2005).
Grandbarbe, L. et al. Delta–Notch signaling controls the generation of neurons/glia from neural stem cells in a stepwise process. Development 130, 1391–1402 (2003).
Jurynczyk, M., Jurewicz, A., Bielecki, B., Raine, C. S. & Selmaj, K. Overcoming failure to repair demyelination in EAE: γ-secretase inhibition of Notch signaling. J. Neurol. Sci. 265, 5–11 (2008).
Nakahara, J., Kanekura, K., Nawa, M., Aiso, S. & Suzuki, N. Abnormal expression of TIP30 and arrested nucleocytoplasmic transport within oligodendrocyte precursor cells in multiple sclerosis. J. Clin. Invest. 119, 169–181 (2009).
Hurlbut, G. D., Kankel, M. W., Lake, R. J. & Artavanis-Tsakonas, S. Crossing paths with Notch in the hyper-network. Curr. Opin. Cell Biol. 19, 166–175 (2007).
Poellinger, L. & Lendahl, U. Modulating Notch signaling by pathway-intrinsic and pathway-extrinsic mechanisms. Curr. Opin. Genet. Dev. 18, 449–454 (2008).
Martinez, A. M. et al. Polyhomeotic has a tumor suppressor activity mediated by repression of Notch signaling. Nature Genet. 41, 1076–1082 (2009).
Bernard, F., Krejci, A., Housden, B., Adryan, B. & Bray, S. J. Specificity of Notch pathway activation: twist controls the transcriptional output in adult muscle progenitors. Development 137, 2633–2642 (2010).
Bray, S. J. Notch signalling: a simple pathway becomes complex. Nature Rev. Mol. Cell Biol. 7, 678–689 (2006).
Kawaguchi, D., Yoshimatsu, T., Hozumi, K. & Gotoh, Y. Selection of differentiating cells by different levels of delta-like 1 among neural precursor cells in the developing mouse telencephalon. Development 135, 3849–3858 (2008).
Johnston, R. J. Jr & Desplan, C. Stochastic neuronal cell fate choices. Curr. Opin. Neurobiol. 18, 20–27 (2008).
Shimojo, H., Ohtsuka, T. & Kageyama, R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron 58, 52–64 (2008). This study elegantly demonstrates the oscillation of Hes1 and other Notch-related genes during development, providing a mechanism for cell fate choices during neurogenesis.
D'Souza, B., Miyamoto, A. & Weinmaster, G. The many facets of Notch ligands. Oncogene 27, 5148–5167 (2008).
Sprinzak, D. et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature 465, 86–90 (2010).
Miller, A. C., Lyons, E. L. & Herman, T. G. cis-Inhibition of Notch by endogenous Delta biases the outcome of lateral inhibition. Curr. Biol. 19, 1378–1383 (2009).
Shikata, Y. et al. Ptch1-mediated dosage-dependent action of Shh signaling regulates neural progenitor development at late gestational stages. Dev. Biol. 349, 147–159 (2011).
Breunig, J. J. et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc. Natl Acad. Sci. USA 105, 13127–13132 (2008).
Lai, K., Kaspar, B. K., Gage, F. H. & Schaffer, D. V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nature Neurosci. 6, 21–27 (2003).
Machold, R. et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 39, 937–950 (2003).
Palma, V. et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132, 335–344 (2005).
Lie, D. C. et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375 (2005).
Estrach, S., Ambler, C. A., Lo Celso, C., Hozumi, K. & Watt, F. M. Jagged 1 is a β-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development 133, 4427–4438 (2006).
Hayward, P. et al. Notch modulates Wnt signalling by associating with Armadillo/β-catenin and regulating its transcriptional activity. Development 132, 1819–1830 (2005).
Espinosa, L., Ingles-Esteve, J., Aguilera, C. & Bigas, A. Phosphorylation by glycogen synthase kinase-3 β down-regulates Notch activity, a link for Notch and Wnt pathways. J. Biol. Chem. 278, 32227–32235 (2003).
Shimizu, T. et al. Stabilized β-catenin functions through TCF/LEF proteins and the Notch/RBP-Jκ complex to promote proliferation and suppress differentiation of neural precursor cells. Mol. Cell. Biol. 28, 7427–7441 (2008).
Aguirre, A., Rubio, M. E. & Gallo, V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 467, 323–327 (2010). This study demonstrates the interplay of Notch and EGFR and shows how the two pathways inhibit each other to establish a balance of progenitor production and stem cell maintenance.
Kuo, C. T. et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell 127, 1253–1264 (2006).
Pece, S., Confalonieri, S., Romano, P. R. & Di Fiore, P. P. NUMB-ing down cancer by more than just a NOTCH. Biochim. Biophys. Acta 1815, 26–43 (2011).
Rasin, M. R. et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nature Neurosci. 10, 819–827 (2007).
Colaluca, I. N. et al. NUMB controls p53 tumour suppressor activity. Nature 451, 76–80 (2008).
Di Marcotullio, L. et al. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nature Cell Biol. 8, 1415–1423 (2006).
Nishimura, T. & Kaibuchi, K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev. Cell 13, 15–28 (2007).
Chigurupati, S. et al. Evidence for altered numb isoform levels in Alzheimer's disease patients and a triple transgenic mouse model. J. Alzheimers Dis. 21 Jan 2011 (doi:10.3233/JAD-2011-101797).
Duan, X., Kang, E., Liu, C. Y., Ming, G. L. & Song, H. Development of neural stem cell in the adult brain. Curr. Opin. Neurobiol. 18, 108–115 (2008).
Johnson, M. A., Ables, J. L. & Eisch, A. J. Cell-intrinsic signals that regulate adult neurogenesis in vivo: insights from inducible approaches. BMB Rep. 42, 245–259 (2009).
Doetsch, F. A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 13, 543–550 (2003).
Shen, Q. et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340 (2004).
Song, H., Stevens, C. F. & Gage, F. H. Astroglia induce neurogenesis from adult neural stem cells. Nature 417, 39–44 (2002).
Palmer, T. D., Willhoite, A. R. & Gage, F. H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 425, 479–494 (2000).
Tavazoie, M. et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3, 279–288 (2008).
Bar, E. E., Lin, A., Mahairaki, V., Matsui, W. & Eberhart, C. G. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am. J. Pathol. 177, 1491–1502 (2010).
Gustafsson, M. V. et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 9, 617–628 (2005). This paper provided an intriguing mechanism for the observation that hypoxia maintains certain stem cell populations. Notably, HIF1α interacts with and stabilizes NICD, promoting receptor signalling.
Pistollato, F. et al. Interaction of hypoxia-inducible factor-1α and notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells 28, 1918–1929 (2010).
Roitbak, T., Surviladze, Z. & Cunningham, L. A. Continuous expression of HIF-1α in neural stem/progenitor cells. Cell. Mol. Neurobiol. 31, 119–133 (2011).
Lim, D. A. & Alvarez-Buylla, A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl Acad. Sci. USA 96, 7526–7531 (1999).
Seri, B., Garcia-Verdugo, J. M., Collado-Morente, L., McEwen, B. S. & Alvarez-Buylla, A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 478, 359–378 (2004).
Nyfeler, Y. et al. Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J. 24, 3504–3515 (2005).
Fabel, K. & Kempermann, G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 10, 59–66 (2008).
Li, G. & Pleasure, S. J. Ongoing interplay between the neural network and neurogenesis in the adult hippocampus. Curr. Opin. Neurobiol. 20, 126–133 (2010).
Ma, D. K., Kim, W. R., Ming, G. L. & Song, H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann. NY Acad. Sci. 1170, 664–673 (2009).
Lugert, S. et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6, 445–456 (2010).In this study the authors identify radial and horizontal stem cells and find that different stem cell pools are recruited depending on the stimulus. Furthermore, they find that some stem cells cannot be activated.
Suh, H. et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1, 515–528 (2007).
Bruel-Jungerman, E., Veyrac, A., Dufour, F., Horwood, J. & Laroche, S. et al. Inhibition of PI3K–Akt signalling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS ONE 4, e7901 (2009).
Snyder, J. S., Glover, L. R., Sanzone, K. M., Kamhi, J. F. & Cameron, H. A. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus, 19, 898–906 (2009).
Carlen, M. et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nature Neurosci. 12, 259–267 (2009).
Jacquet, B. V. et al. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development 136, 4021–4031 (2009).
Nomura, T., Goritz, C., Catchpole, T., Henkemeyer, M. & Frisen, J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell 7, 730–743 (2010). This study demonstrates an incredible phenotypic plasticity of adult neural stems and niche cell types.
Deng, W., Aimone, J. B. & Gage, F. H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature Rev. Neurosci. 11, 339–350 (2010).
Milano, J. et al. Modulation of notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 82, 341–358 (2004).
Hicks, C. et al. A secreted Delta1-Fc fusion protein functions both as an activator and inhibitor of Notch1 signaling. J. Neurosci. Res. 68, 655–667 (2002).
Beckstead, B. L. et al. Methods to promote Notch signaling at the biomaterial interface and evaluation in a rafted organ culture model. J. Biomed. Mater. Res. A 91, 436–446 (2009).
Breunig, J. J., Arellano, J. I., Macklis, J. D. & Rakic, P. Everything that glitters isn't gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell 1, 612–627 (2007).
Mason, H. A., Rakowiecki, S. M., Gridley, T. & Fishell, G. Loss of notch activity in the developing central nervous system leads to increased cell death. Dev. Neurosci. 28, 49–57 (2006).
Hori, K. et al. A nonclassical bHLH Rbpj transcription factor complex is required for specification of GABAergic neurons independent of Notch signaling. Genes Dev. 22, 166–178 (2008).
Saint Just Ribeiro, M. & Wallberg, A. E. Transcriptional mechanisms by the coregulator MAML1. Curr. Protein Pept. Sci. 10, 570–576 (2009).
Zhang, L. & Gallagher, P. J. Mind bomb 1 regulation of cFLIP interactions. Am. J. Physiol. Cell Physiol. 297, C1275–C1283 (2009).
Zhang, X. P. et al. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol. Cell. Biochem. 307, 101–108 (2008).
Purow, B. W. et al. Notch-1 regulates transcription of the epidermal growth factor receptor through p53. Carcinogenesis 29, 918–925 (2008).
Huang, P. H., Xu, A. M. & White, F. M. Oncogenic EGFR signaling networks in glioma. Sci. Signal 2, re6 (2009).
Chen, J. et al. Characterization of human LNX, a novel ligand of Numb protein X that is downregulated in human gliomas. Int. J. Biochem. Cell Biol. 37, 2273–2283 (2005).
Nakamura, H. et al. Identification of a human homolog of the Drosophila neuralized gene within the 10q25.1 malignant astrocytoma deletion region. Oncogene 16, 1009–1019 (1998).
Rehman, A. O. & Wang, C. Y. Notch signaling in the regulation of tumor angiogenesis. Trends Cell Biol. 16, 293–300 (2006).
Zheng, X. et al. Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc. Natl Acad. Sci. USA 105, 3368–3373 (2008).
Stockhausen, M. T., Kristoffersen, K. & Poulsen, H. S. The functional role of Notch signaling in human gliomas. Neuro Oncol. 12, 199–211 (2010).
Wang, J. et al. Notch promotes radioresistance of glioma stem cells. Stem Cells 28, 17–28 (2010).
Fan, X. et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 64, 7787–7793 (2004).
Chen, J. et al. Inhibition of Notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer 1, 822–835 (2010).
Miles, D. K. & Kernie, S. G. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus 18, 793–806 (2008).
Irvin, D. K., Zurcher, S. D., Nguyen, T., Weinmaster, G. & Kornblum, H. I. Expression patterns of Notch1, Notch2, and Notch3 suggest multiple functional roles for the Notch-DSL signaling system during brain development. J. Comp. Neurol. 436, 167–181 (2001).
Murphy, P. A. et al. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc. Natl Acad. Sci. USA 105, 10901–10906 (2008).
Irvin, D. K., Nakano, I., Paucar, A. & Kornblum, H. I. Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J. Neurosci. Res. 75, 330–343 (2004).
Yoon, K. J. et al. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron 58, 519–531 (2008). This fascinating study demonstrates that daughter progenitor cells can directly provide ligand stimulation to the Notch receptor present on neural stem cells.
Shutter, J. R. et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 14, 1313–1318 (2000).
Lavado, A., Lagutin, O. V., Chow, L. M., Baker, S. J. & Oliver, G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 8, e1000460 (2010).
Kurisu, J., Fukuda, T., Yokoyama, S., Hirano, T. & Kengaku, M. Polarized targeting of DNER into dendritic plasma membrane in hippocampal neurons depends on endocytosis. J. Neurochem. 113, 1598–1610 (2010).
Acknowledgements
J.L.A. would like to thank the Medical Science Training Program (MSTP) at both the University of Texas Southwestern Medical Centre, at Dallas, Texas, USA, and Mount Sinai School of Medicine, New York, New York, USA, as well as the US National Institute of Mental Health (NIMH) and National Institute on Drug Abuse (NIDA) for their support. J.J.B. was supported by the Connecticut Stem Cell Research Grant Program during the preparation of this manuscript and is currently supported by the Cedars-Sinai Regenerative Medicine Institute, Los Angeles, California, USA. A.J.E. is supported by grants from the US National Institutes of Health (NIH), including grants from NIDA (DA016765, DA016765-07S, DA023555) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK079328), the National Alliance for Research on Schizophrenia and Depression, the National Aeronautics and Space Administration, and the Norwegian Department of Public Health. P.R. is supported by grants from the US National Institute of Neurological Disorders and Stroke (NINDS), NIDA and the Kavli Institute of Neuroscience at Yale University School of Medicine, New Haven, Connecticut, USA.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Ables, J., Breunig, J., Eisch, A. et al. Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci 12, 269–283 (2011). https://doi.org/10.1038/nrn3024
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3024
This article is cited by
-
Effect of Octamer-Binding Transcription Factor 4 Overexpression on the Neural Induction of Human Dental Pulp Stem Cells
Stem Cell Reviews and Reports (2024)
-
The Notch-mediated circuitry in the evolution and generation of new cell lineages: the tooth model
Cellular and Molecular Life Sciences (2023)
-
Regulatory role of melatonin in Notch1 signaling pathway in cerebral cortex of Aβ1−42-induced Alzheimer’s disease rat model
Molecular Biology Reports (2023)
-
Signaling mechanisms involved in the regulation of remyelination in multiple sclerosis: a mini review
Journal of Molecular Medicine (2023)
-
EGFL7 loss correlates with increased VEGF-D expression, upregulating hippocampal adult neurogenesis and improving spatial learning and memory
Cellular and Molecular Life Sciences (2023)