Key Points
-
Most of what we understand about microbial energy metabolism derives from the study of cultured organisms that poorly represent those in low-energy settings, both in phylogeny and physiological state.
-
A large fraction of bacteria and archaea on Earth live in the deep subsurface, where fluxes of energy can be orders of magnitude lower than in our surface world.
-
Organisms in low-energy environments catabolize and turn over biomass 105–106-fold more slowly than those operating near Vmax in culture, and subsist with energy fluxes 104-fold lower than culture-based estimates of maintenance energy.
-
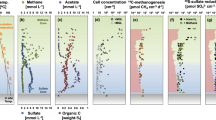
The calculated mean turnover times of cell biomass in the sub-seafloor deep biosphere is a few hundred to a few thousand years: that is, 100–1,000 times slower than in surface sediments.
-
Mean cell-specific rates of metabolism in subsurface microbial communities scatter around 10−4 to 10−3 fmol cell−1 d−1.
-
This range of metabolic rates probably reflects the 'basal power requirement': that is, the energy turnover rate per cell or per unit biomass associated with the minimal complement of functions required to sustain a metabolically active state of the cell.
Abstract
A great number of the bacteria and archaea on Earth are found in subsurface environments in a physiological state that is poorly represented or explained by laboratory cultures. Microbial cells in these very stable and oligotrophic settings catabolize 104- to 106-fold more slowly than model organisms in nutrient-rich cultures, turn over biomass on timescales of centuries to millennia rather than hours to days, and subsist with energy fluxes that are 1,000-fold lower than the typical culture-based estimates of maintenance requirements. To reconcile this disparate state of being with our knowledge of microbial physiology will require a revised understanding of microbial energy requirements, including identifying the factors that comprise true basal maintenance and the adaptations that might serve to minimize these factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Whitman, W. B., Coleman, D. C. & Wiebe, W. J. Prokaryotes: the unseen majority. Proc. Natl Acad. Sci. USA 95, 6578–6583 (1998). An early effort to estimate the magnitude and extent of the deep biosphere.
Kallmeyer, J., Pockalny, R., Adhikari, R. R., Smith, D. C. & D'Hondt, S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc. Natl Acad. Sci. USA 109, 16213–16216 (2012).
DeLong, J. P., Okie, J. G., Mosesa, M. E., Siblyd, R. M. & Brown, J. H. Shifts in metabolic scaling, production, and efficiency across major evolutionary transitions of life. Proc. Natl Acad. Sci. USA 107, 12941–12945 (2010).
Kempes, C., Dutkiewicz, S. & Follows, M. Growth, metabolic partitioning, and the size of microorganisms. Proc. Natl Acad. Sci. USA 109, 495–500 (2012).
Makarieva, A. M., Gorshkov, V. G. & Li, B.-L. Energetics of the smallest: do bacteria breathe at the same rate as whales? Proc. R. Soc. B 272, 2219–2224 (2005).
Makarieva, A. M. et al. Mean mass-specific metabolic rates are strikingly similar across life's major domains: evidence for life's metabolic optimum. Proc. Natl Acad. Sci. USA 105, 16994–16999 (2008).
Mason, M. M. A comparison of the maximal growth rates of various bacteria under optimal conditions. J. Bacteriol. 29, 103–110 (1935).
Finkel, S. E. Long-term survival during stationary phase: evolution and the GASP phenotype. Nature Rev. Microbiol. 4, 113–120 (2006). An excellent review of extended stationary phase and the GASP phenotype.
Zambrano, M. M., Siegele, D. A., Almiron, M., Tormo, A. & Kolter, R. Microbial competition: E. coli mutants that take over stationary phase cultures. Science 259, 1757–1760 (1993).
Finkel, S. E. & Kolter, R. Evolution of microbial diversity during prolonged starvation. Proc. Natl Acad. Sci. USA 96 4023–4027 (1999).
Steinhaus, E. A. & Birkeland, J. M. Studies on the life and death of bacteria. I. The senescent phase in aging cultures and the probable mechanisms invovled. J. Bacteriol. 38, 249–261 (1939).
Zinser, E. R. & Kolter, R. E. coli evolution during stationary phase. Res. Microbiol. 155, 328–336 (2004).
Zinser, E. R. & Kolter, R. Mutations enhancing amino acid catabolism confer a growth advantage in stationary phase. J. Bacteriol. 181, 5800–5807 (1999).
Zinser, E. R. & Kolter, R. Prolonged stationary phase incubation selects for lrp mutants in E. coli K-12. J. Bacteriol. 182, 4361–4365 (2000).
Farrell, M. J. & Finkel, S. E. The growth advantage in stationary phase phenotype conferred by rpoS mutations is dependent on the pH and nutrient environment. J. Bacteriol. 185, 7044–7052 (2003).
Røy, H. et al. Aerobic microbial respiration in 86-million-year-old deep-sea red clay. Science 336, 922–925 (2012). The observation of aerobic microbial respiration in 86-million-year-old sediments in one of Earth's most oligotrophic settings.
Russell, J. B. & Cook, G. M. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol. Rev. 59, 48–62 (1995). An excellent overview of energy metabolism, maintenance energy and energy spilling.
Tempest, D. W. & Neijssel, O. M. The status of Y ATP and maintenance energy as biologically interpretable phenomena. Annu. Rev. Microbiol. 38, 459–486 (1984).
van Bodegom, P. Microbial maintenance: a critical review on its quantification. Microb. Ecol. 53, 513–523 (2007).
Bauchop, T. & Eldsen, S. R. The growth of microorganisms in relation to their energy supply. J. Gen. Microbiol. 23, 457–469 (1960).
Stouthamer, A. H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Leeuwenhoek 39, 545–565 (1973).
Stouthamer, A. H. & Bettenhaussen, C. W. Utilizatoin of energy for growth and maintenance in continuous and batch cultures of microorganisms. Biochim. Biophys. Acta 301, 53–70 (1973).
Stouthamer, A. H. in International Review of Biochemistry and Microbial Biochemistry Vol. 21 (ed. Quayle, J. R.) 1–47 (Univ. Park Press, 1979).
Pirt, S. J. The maintenance energy of bacteria in growing cultures. Proc. R. Soc. B 163, 224–231 (1965).
Pirt, S. J. Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch. Microbiol. 133, 300–302 (1982).
Herbert, D., Elsworth, R. & Telling, R. C. The continuous culture of bacterial: a theoretical and experimental study. J. Gen. Microbiol. 14, 601–622 (1956).
Novick, A. & Szilard, L. Description of the chemostat. Science 112, 715–716 (1950).
Tijhuis, L., van Loosdrecht, M. C. M. & Heijnen, J. J. A thermodynamically based correlation for maintenance Gibbs energy requirements in aerobic and anaerobic chemotrophic growth. Biotechnol. Bioengineer. 42, 509–519 (1993).
Morita, R. Bacteria in Oligotrophic Environments (Chapman & Hall, 1997).
Morita, R. Is H2 the universal energy source for long-term survival? Microb. Ecol. 38, 307–320 (2000).
Scholten, J. C. M. & Conrad, R. Energetics of syntrophic propionate oxidation in defined batch and chemostat cocultures. Appl. Environ. Microbiol. 66, 2934–2942 (2000).
Tappe, W. et al. Maintenance energy demand and starvation recovery dynamics of Nitrosomonas europaea and Nitrobacter winogradskyi cultivated in a retentostat with complete biomass retention. Appl. Environ. Microbiol. 65, 2471–2477 (1999).
Niejssel, O. M. & Tempest, D. W. The role of energy-spilling reactions in the growth of Klebsiella aerogenes NCTC 418 in aerobic chemostat culture. Arch. Microbiol. 110, 305–311 (1976).
Cook, G. M. & Russell, J. B. Energy spilling reactions of Streptococcus bovis and resistance of its membrane to proton conductance. Appl. Environ. Microbiol. 60, 1942–1948 (1994).
Russell, J. B. & Strobel, H. J. ATPase-dependent energy spilling by the ruminal bacterium, Streptococcus bovis. Arch. Microbiol. 153, 378–383 (1990).
Groisman, A. et al. A microfluidic chemostat for experiments with bacterial and yeast cells. Nature Methods 2, 685–689 (2005).
Lee, K. S., Boccazzi, P., Sinskey, A. J. & Ram, R. J. Microfluidic chemostat and turbidostat with flow rate, oxygen, and temperature control for dynamic continuous culture. Lab. Chip 11, 1730–1739 (2011).
Lin, B., Westerhoff, H. V. & Röling, W. F. M. How Geobacteraceae may dominate subsurface biodegradation: physiology of Geobacter metallireducens in slow-growth habitat-simulating retentostats. Environ. Microbiol. 11, 2425–2433 (2009).
Tappe, W., Tomaschewski, C., Rittershaus, S. & Groeneweg, J. Cultivation of nitrifying bacteria in the retentostat, a simple fermenter with internal biomass retention. FEMS Microbiol. Ecol. 19, 47–52 (1996).
Girguis, P. R., Cozen, A. E. & DeLong, E. F. Growth and population dynamics of anaerobic methane-oxidizing archaea and sulfate-reducing bacteria in a continuous-flow bioreactor. Appl. Environ. Microbiol. 71, 3725–3733 (2005).
Imachi, H. et al. Cultivation of methanogenic community from subseafloor sediments using a continuous-flow bioreactor. ISME J. 5, 1913–1925 (2011).
Parkes, R. J. et al. Bacterial biomass and activity in deep sediment. Layers from the Peru Margin [and Discussion]. Phil. Trans. R. Soc. Lond. A 331, 139–153 (1990). One of the earliest demonstrations of the presence of active microorganisms in deep sediments.
Joye, S. B. et al. The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps. Chem. Geol. 205, 219–238 (2004).
Treude, T. et al. Anaerobic oxidation of methane and sulfate reduction along the Chilean continental margin. Geochim. Cosmochim. Acta 69, 2767–2779 (2005).
Parkes, R. et al. Deep sub-seafloor prokaryotes stimulated at interfaces over geological time. Nature 436, 390–394 (2005).
Boudreau, B. P. Diagenetic Models and their Implementation (Springer, 1997).
Arndt, S., Hetzel, A. & Brumsack, H. J. Evolution of organic matter degradation in Cretaceous black shales inferred from authigenic barite: a reaction-transport model. Geochim. Cosmochim. Acta 73, 2000–2022 (2009).
Wang, G., Spivack, A. J., Rutherford, S., Manorc, U. & D'Hondt, S. Quantification of co-occurring reaction rates in deep subseafloor sediments. Geochim. Cosmochim. Acta 72, 3479–3488 (2008).
Phelps, T. J., Murphy, E. M., Pfiffner, M. & White, D. C. Factors influencing the abundance and metabolic capacities of microorganisms in eastern coastal-plain sediments. Microb. Ecol. 28, 335–349 (1994).
Wheat, C. G. & Fisher, A. T. Seawater recharge along an eastern bounding fault in Middle Valley, northern Juan de Fuca Ridge. Geophys. Res. Lett. 34, L20602 (2007).
Chapelle, F. H. & Lovley, D. R. Rates of microbial metabolism in deep coastal plain aquifers. Appl. Environ. Microbiol. 56, 1865–1874 (1990).
Kallmeyer, J. Detection and quantification of microbial cells in subsurface sediments. Adv. Appl. Microbiol. 76, 79–103 (2011).
Morono, Y., Terada, T., Masui, N. & Inagaki, F. Discriminative detection and enumeration of microbial life in marine subsurface sediments. ISME J. 3, 503–511 (2009).
Kallmeyer, J., Smith, D. C., Spivack, A. J. & D'Hondt, S. New cell extraction procedure applied to deep subsurface sediments. Limnol. Oceanogr. Methods 6, 236–245 (2008).
Parkes, R. J., Cragg, B. A. & Wellsbury, P. Recent studies on bacterial populations and processes in subseafloor sediments: a review. Hydrogeol. Rev. 8, 11–28 (2000).
D'Hondt, S. et al. Subseafloor sedimentary life in the South Pacific Gyre. Proc. Natl Acad. Sci. USA 106, 11651–11656 (2009).
Cockell, C. S. et al. Impact disruption and recovery of the deep subsurface biosphere. Astrobiology 12, 231–246 (2012).
Amann, R. I. Fluorescently labeled, ribosomal-RNA-targeted oligonucleotide probes in the study of microbial ecology. Mol. Ecol. 4, 543–553 (1995).
Wagner, M. et al. Functional marker genes for identification of sulfate-reducing prokaryotes. Methods Enzymol. 397, 469–489 (2005).
Teske, A. & Sørensen, K. B. Uncultured archaea in deep marine subsurface sediments: have we caught them all? ISME J. 2, 3–8 (2008).
Lipp, J. S., Morono, Y., Inagaki, F. & Hinrichs, K.-U. Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature 454, 991–994 (2008).
Logemann, J. et al. A laboratory experiment of intact polar lipid degradation in sandy sediments. Biogeosciences 8, 2547–2560 (2011).
Schouten, S., Middleburg, J. J., Hopmans, E. C. & Damsté, J. S. S. Fossilization and degradation of intact polar lipids in deep subsurface sediments: a theoretical approach. Geochim. Cosmochim. Acta 74, 3806–3814 (2010).
D'Hondt, S., Rutherford, S. & Spivack, A. J. Metabolic activity of subsurface life in deep-sea sediments. Science 295, 2067–2070 (2002). An early observation of low process rates in deep sediments.
Lomstein, B. A. et al. Endospore abundance, microbial growth and necromass turnover in deep sub-seafloor sediment. Nature 484, 101–104 (2012).
Bada, J. L. Racemization of amino acids in nature. Interdiscip. Sci. Rev. 7, 30–46 (1982).
Biddle, J. F. et al. Heterotrophic Archaea dominate sedimentary subsurface ecosystems off Peru. Proc. Natl Acad. Sci. USA 103, 3846–3851 (2006).
Holmkvist, L. et al. Sulfate reduction below the sulfate-methane transition in Black Sea sediments. Deep Sea Res. Part I Oceonogr. Res. Pap. 58, 493–504 (2011).
Holmkvist, L., Ferdelman, T. G. & Jørgensen, B. B. A cryptic sulfur cycle driven by iron in the methane zone of marine sediment (Aarhus Bay, Denmark). Geochim. Cosmochim. Acta 75, 3581–3599 (2011).
Leloup, J. Sulfate-reducing bacteria in marine sediment (Aarhus Bay, Denmark): abundance and diversity related to geochemical zonation. Environ. Microbiol. 11, 1278–1291 (2009).
Leloup, J. et al. Diversity and abundance of sulfate-reducing microorganisms in the sulfate and methane zones of a marine sediment, Black Sea. Environ. Microbiol. 9, 131–142 (2007).
Ravenschlag, K. et al. Community structure, cellular rRNA content and activity of sulfate-reducing bacteria in marine Arctic sediments. Appl. Environ. Microbiol. 66, 3592–3602 (2000).
Sahm, K., MacGregor, B. J., Jørgensen, B. B. & Stahl, D. A. Sulphate reduction and vertical distribution of sulphate-reducing bacteria quantified by rRNA slot-blot hybridization in a coastal marine sediment. Environ. Microbiol. 1, 65–74 (1999).
Widdel, F. in Biology of Anaerobic Microorganisms (ed. Zehnder, A. J. B.) 469–586 (Wiley-Interscience, 1988).
McCollom, T. M. & Amend, J. P. A thermodynamic assessment of energy requirements for biomass synthesis by chemolithoautotrophic microorganisms in oxic and anoxic environments. Geobiology 3, 135–144 (2005).
Detmers, J., Brüchert, V., Habicht, K. S. & Kuever, J. Diversity of sulfur isotope fractionations by sulfate-reducing prokaryotes. Appl. Environ. Microbiol. 67, 888–894 (2001).
Knoblauch, C. & Jørgensen, B. B. Effect of temperature on sulfate reduction, growth rate, and growth yield in five psychrophilic sulfate-reducing bacteria from Arctic sediments. Environ. Microbiol. 1, 457–467 (1999).
Knoblauch, C., Jørgensen, B. B. & Harder, J. Community size and metabolic rates of psychrophilic sulfate-reducing bacteria in Arctic marine sediments. Appl. Environ. Microbiol. 65, 4230–4233 (1999).
Tarpgaard, I. H., Boetius, A. & Finster, K. Desulfobacter psychrotolerans sp. nov., a new psychrotolerant sulfate-reducing bacterium and descriptions of its physiological response to temperature changes. Antonie Leeuwenhoek 89, 109–124 (2006).
Schippers, A. & Neretin, L. N. Quantification of microbial communities in near-surface and deeply buried marine sediments on the Peru continental margin using real-time PCR. Environ. Microbiol. 8, 1251–1260 (2006).
Lin, L. H. et al. Long-term sustainability of a high-energy, low-diversity crustal biome. Science 314, 479–482 (2006).
Price, B. & Sowers, T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc. Natl Acad. Sci. USA 101, 4631–4636 (2004).
Berg, H. C. The rotary motor of bacterial flagella. Biochemistry 72, 19–54 (2003).
Fenchel, T. Motility of bacteria in sediments. Aquat. Microb. Ecol. 51, 23–30 (2008).
Lennon, J. T. & Jones, S. E. Microbial seed banks: the ecological and evolutionary implications of dormancy. Nature Rev. Microbiol. 119, 119–130 (2011).
Johnson, S. S. et al. Ancient bacteria show evidence of DNA repair. Proc. Natl Acad. Sci. USA 104, 14401–14405 (2007).
Hammes, F., Berney, M. & Egli, T. Cultivation-independent assessment of bacterial viability. Adv. Biochem. Engineer. Biotechnol. 124, 123–150 (2012).
Schippers, A. et al. E. Prokaryotic cells of the deep sub-seafloor biosphere identified as living bacteria. Nature 433, 861–864 (2005).
Morono, Y. et al. Carbon and nitrogen assimilation in deep subseafloor microbial cells. Proc. Natl Acad. Sci. USA 108, 18295–18300 (2011). A demonstration that metabolic activity is rapidly induced in most sub-seafloor cells.
D'Hondt, S. et al. Distributions of microbial activities in deep subseafloor sediments. Science 306, 2216–2221 (2004).
D'Hondt, S. L. et al. Proceedings of the Ocean Drilling Program Vol. 201 (ed. Peters, L. L.) [online], http://www-odp.tamu.edu/publications/201_IR/201TOC.HTM (2003).
Schrum, H. N., Spivack, A. J., Kastner, M. & D'Hondt, S. Sulfate-reducing ammonium oxidation: a thermodynamically feasible metabolic pathway in subseafloor sediment. Geology 37, 939–942 (2009).
Lin, L. H. et al. Radiolytic H2 in continental crust: nuclear power for deep subsurface microbial communities. Geochem. Geophys. Geosystems 6, Q07003 (2005).
Blair, C. C., D'Hondt, S., Spivack, A. J. & Kingsley, R. H. Radiolytic hydrogen and microbial respiration in subsurface sediments. Astrobiology 7, 951–970 (2007).
Rang, C. U., Peng, A. Y. & Chao, L. Temporal dynamics of bacterial aging and rejuvenation. Curr. Biol. 21, 1813–1816 (2011).
Edgcomb, V. P., Beaudoin, D., Gast, R., Biddle, J. F. & Teske, A. Marine subsurface eukaryotes: the fungal majority. Environ. Microbiol. 13, 172–183 (2011).
Danovaro, R. et al. Major viral impact on the functioning of deep-sea ecosystems. Nature 454, 1084–1087 (2008).
Engelhardt, T., Sahlberg, M., Cypionka, H. & Engelen, B. Induction of prophages from deep-subseafloor bacteria. Environ. Microbiol. Rep. 3, 459–465 (2011).
Middelboe, M., Glud, R. N. & Filippini, M. Viral abundance and activity in the deep sub-seafloor biosphere. Aquat. Microb. Ecol. 63, 1–8 (2011).
Brinton, K. L. F., Tsapin, A., Gilichinsky, D. & McDonald, G. D. Aspartic acid racemization and age–depth relationships for organic carbon in Siberian permafrost. Astrobiology 2, 77–82 (2002).
Lindahl, T. & Karlstrom, O. Heat-induced depyrimidination of deoxyribonucleic acid in neutral solution. Biochemistry 12, 5151–5154 (1973).
Lever, M. A. Acetogenesis in the energy starved deep biosphere – a paradox? Front. Microbiol. 2, 1–14 (2012).
Schink, B. in Biology of Anaerobic Microorganisms (ed. Zehnder, A. J. B.) 771–846 (Wiley-Interscience, 1988).
Schink, B. & Stams, A. J. M. in The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community (eds Dworkin, M. et al.) 309–335 (Springer, 2002).
Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism. Nature 191, 144–148 (1961).
van den Vossenberg, J. L. C. M., Ubbink-Kok, T., Elferink, M. G. L., Driessen, A. J. M. & Konings, W. N. Ion permability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol. Microbiol. 18, 925–932 (1995).
Van den Vossenberg, J. L. C. M., Driessen, A. J. M. & Konings, W. N. in Cell and Molecular Response to Stress (eds Storey, K. B. & Storey, J. M.) 71–88 (Elsevier, 2000).
Schlegel, K., Leone, V., Faraldo-Gómez, J. D. & Müller, V. Promiscuous archaeal ATP synthase concurrently coupled to Na+ and H+ translocation. Proc. Natl Acad. Sci. USA 109, 947–952 (2012).
Inagaki, F. et al. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc. Natl Acad. Sci. USA 103, 2815–2820 (2006).
Sørensen, K. B. & Teske, A. Stratified communities of active archaea in deep marine subsurface sediments. Appl. Environ. Microbiol. 72, 4596–4603 (2006).
Schippers, A., Köweker, G., Höft, C. & Teichert, M. A. Quantification of microbial communities in forearc sediment basins off Sumatra. Geomicrobiol. J. 27, 170–182 (2010).
Kubo, K. et al. Archaea of the Miscellaneous Crenarchaeotal Group are abundant, diverse, and widespread in marine sediments. ISME J. 6, 1949–1965 (2012).
Schippers, A., Köck, D., Höft, C., Koweker, G. & Siegert, M. Quantification of microbial communities in subsurface marine sediments of the Black Sea and off Namibia. Front. Microbiol. 3, 1–11 (2012).
Valentine, D. L. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nature Rev. Microbiol. 5, 316–323 (2007).
Van de Vossenberg, J. L. C. M. Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol. Microbiol. 18, 925–932 (1995).
Takano, Y. et al. Sedimentary membrane lipids recycled by deep-sea benthic archaea. Nature Geosci. 3, 858–861 (2010).
Hoehler, T. M., Alperin, M. J., Albert, D. B. & Martens, C. S. Apparent minimum free energy requirements for methanogenic archaea and sulfate-reducing bacteria in an anoxic marine sediment. FEMS Microbiol. Ecol. 38, 33–41 (2001).
Shade, A. et al. Culturing captures members of the soil rare biosphere. Environ. Microbiol. 14, 2247–2252 (2012).
Rappe, M. S. & Giovannoni, S. J. The uncultured microbial majority. Annu. Rev. Microbiol. 57, 369–394 (2003).
Stepanauskas, R. & Sieracki, M. E. Matching phylogeny and metabolism in the uncultured marine bacteria, on cell at a time. Proc. Natl Acad. Sci. USA 104, 9052–9057 (2007).
Schrödinger, E. What is Life? The Physical Aspect of the Living Cell (Cambridge Univ. Press, 1944).
Duclaux, E. Traite de Microbiologie (Masson, 1900).
Marr, A. G., Nilson, E. H. & Clark, D. J. The maintenance requirement of Escherichia coli. Ann. NY Acad. Sci. 102, 536–548 (1963).
Niejssel, O. M. & Tempest, D. W. Bioenergetic aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon-sufficient culture. Arch. Microbiol. 107, 215–221 (1976).
Bulthuis, B. A., Frankena, J., Koningstein, G. M., van Verseveld, H. W. & Stouthamer, A. H. A comparison between aerobic growth of Bacillus licheniformis in continuous culture and partial-recycling fermentor, with contributions to the discussion on maintenance energy demand. Arch. Microbiol. 152, 499–507 (1989).
Smith, D. C. et al. Methods for quantifying potential microbial contamination during deep ocean coring. ODP Technical Note 28 (doi:10.2973/odp.tn.28.2000) (2000).
Lever, M. A. et al. Trends in basalt and sediment core contamination during IODP Expedition 301. Geomicrobiol. J. 23, 517–530 (2006).
Edwards, K. J., Becker, K. & Colwell, F. S. The deep, dark energy biosphere: intraterrestrial life on Earth. Annu. Rev. Earth Planet. Sci. 40, 551–568 (2012).
Fry, J. C. et al. Prokaryotic populations and activities in an interbedded coal deposit, including a previously deeply buried section (1.6-2.3km) above ∼150 Ma basement rock. Geomicrobiol. J. 26, 163–178 (2009).
Sahm, K., Macgregor, B. J., Jørgensen, B. B. & Stahl, D. A. Sulfate reduction and vertical distribution of sulphate-reducing bacteria quantified by rRNA slot-blot hybridization in a coastal marine sediment. Environ. Microbiol. 1, 65–74 (1999).
Acknowledgements
T.M.H. is supported by the NASA Astrobiology Institute and Exobiology Program, and B.B.J is supported by the Danish National Research Foundation, the German Max Planck Society and the European Research Council. The authors thank M. A. Lever, H. Røy, A. Schippers and an anonymous reviewer for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Bo Barker Jørgensen's homepage
Second International Workshop on Microbial Life under Extreme Energy Limitation
Glossary
- Deep biosphere
-
The set of ecosystems and their organisms living beneath the upper few metres of the solid earth surface.
- Extended stationary phase
-
A phase of the batch culture life cycle characterized by the persistence of a small fraction of cells for months to years beyond the death of the majority of the culture, without new addition of substrate.
- Y ATP
-
Cellular growth yield normalized to ATP consumption.
- Basal power requirement
-
Energy turnover rate per cell or per unit biomass associated with the minimal complement of functions required to sustain a metabolically active state of the cell.
- Mean cell-specific metabolic rates
-
Estimate of average cellular metabolic rate among a whole community of cells obtained by measurement of bulk metabolic process rates and cell numbers.
- Primary productivity
-
The formation of living organic biomass from carbon dioxide through the process of photosynthesis or chemosynthesis.
- Reaction-transport modelling
-
Calculation of metabolic process rates based on steady-state concentration-depth profiles and calculated metabolite fluxes.
- Power law
-
A mathematical relationship between two quantities describing how one quantity, c, varies as a power of another quantity, z: for example, c = A × z−b, in which c could be cell density, z sediment depth (z> >0), and A and b constants.
- Bioturbated sediment
-
The uppermost part of the seabed that is physically reworked by animals.
- Gyre
-
A large system of rotating ocean currents, such as those involved with large wind movements at mid-latitudes on the northern and southern Pacific and Atlantic Ocean.
- Amino acid racemization
-
Conversion of one stereoisomer of an amino acid to another stereoisomer that is a mirror image of the former.
- Depurination
-
An alteration of DNA in which the purine base (adenine or guanine) is lost from the deoxyribose sugar by hydrolysis of the β-N-glycosidic link between them.
Rights and permissions
About this article
Cite this article
Hoehler, T., Jørgensen, B. Microbial life under extreme energy limitation. Nat Rev Microbiol 11, 83–94 (2013). https://doi.org/10.1038/nrmicro2939
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2939
This article is cited by
-
The structure and development of Loess Critical Zone and its soil carbon cycle
Carbon Neutrality (2024)
-
The microbial dark matter and “wanted list” in worldwide wastewater treatment plants
Microbiome (2023)
-
May microbial ecological baseline exist in continental groundwater?
Microbiome (2023)
-
Accessing the energy-limited and sparsely populated deep biosphere: achievements and ongoing challenges of available technologies
Progress in Earth and Planetary Science (2023)
-
Metagenomic profiles of archaea and bacteria within thermal and geochemical gradients of the Guaymas Basin deep subsurface
Nature Communications (2023)