Key Points
-
Anaplasma spp. and Ehrlichia spp. cause several emerging human infectious diseases. They are obligate intracellular bacteria that reside in cells of haematopoietic origin in mammals and also in tick cells. This Review focuses on recent A. phagocytophilum and E. chaffeensis studies related to their pathogenesis, observed primarily from the bacterial perspective.
-
A. phagocytophilum and E. chaffeensis lack genes for the biosynthesis of the lipopolysaccharide and peptidoglycan that activate host leukocytes. Instead, they acquire host cholesterol from the low-density-lipoprotein uptake pathway.
-
Caveolae-mediated endocytosis directs A. phagocytophilum and E. chaffeensis to an intracellular compartment, or inclusion, that does not acquire components of NADPH oxidase nor show similarity to late endosomes or lysosomes. E. chaffeensis inclusions retain early-endosome characteristics, whereas A. phagocytophilum inclusions acquire early-autophagosome characteristics.
-
A. phagocytophilum and E. chaffeensis both have small genomes (approximately 1.2–1.5 Mb) with a low coding capacity for proteins involved in central intermediary metabolism and amino acid synthesis. However, both retained genes for aerobic respiration and for the biosynthesis of all of the necessary nucleotides and most vitamins and cofactors.
-
In A. phagocytophilum there is an expansion of the OMP1–P44 superfamily encoding outer-membrane proteins that are unique to the family Anaplasmataceae. Some of these proteins have been shown to be porins.
-
In cell culture A. phagocytophilum and E. chaffeensis undergo developmental stages known as dense-cored cells and reticulate cells. The ApxR and EcxR transcription factors are unique to the family Anaplasmataceae, and three two-component systems are involved in regulating the intracellular development of the species in this family.
-
Expression of the type IV secretion system is developmentally regulated, and two secreted effectors have been shown to regulate protein tyrosine kinases and cellular apoptosis.
Abstract
Anaplasma spp. and Ehrlichia spp. cause several emerging human infectious diseases. Anaplasma phagocytophilum and Ehrlichia chaffeensis are transmitted between mammals by blood-sucking ticks and replicate inside mammalian white blood cells and tick salivary-gland and midgut cells. Adaptation to a life in eukaryotic cells and transmission between hosts has been assisted by the deletion of many genes that are present in the genomes of free-living bacteria (including genes required for the biosynthesis of lipopolysaccharide and peptidoglycan), by the acquisition of a cholesterol uptake pathway and by the expansion of the repertoire of genes encoding the outer-membrane porins and type IV secretion system. Here, I review the specialized properties and other adaptations of these intracellular bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Theiler, A. Anaplasma marginale (gen. and spec. nov.) The marginal points in the blood of cattle suffering from specific disease. Transvaal S. Afr. Rep.Vet. Bacteriol. Dept. Agr. 1908–1909, 7–64 (1910).
Thomas, R. J., Dumler, J. S. & Carlyon, J. A. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev. Anti Infect. Ther. 7, 709–722 (2009).
Paddock, C. D. & Childs, J. E. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16, 37–64 (2003).
Bakken, J. S. & Dumler, S. Human granulocytic anaplasmosis. Infect. Dis. Clin. North Am. 22, 433–448 (2008).
Olano, J. P. & Walker, D. H. Human ehrlichioses. Med. Clin. North Am. 86, 375–392 (2002).
Gardner, S. L., Holman, R. C., Krebs, J. W., Berkelman, R. & Childs, J. E. National surveillance for the human ehrlichioses in the United States, 1997–2001, and proposed methods for evaluation of data quality. Ann. N.Y. Acad. Sci. 990, 80–89 (2003).
Newton, P. N. et al. Sennetsu neorickettsiosis: a probable fish-borne cause of fever rediscovered in Laos. Am. J. Trop. Med. Hyg. 81, 190–194 (2009).
Perez, M., Bodor, M., Zhang, C., Xiong, Q. & Rikihisa, Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N.Y. Acad. Sci. 1078, 110–117 (2006).
Perez, M., Rikihisa, Y. & Wen, B. Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J. Clin. Microbiol. 34, 2133–2139 (1996).
Allsopp, M. T., Louw, M. & Meyer, E. C. Ehrlichia ruminantium: an emerging human pathogen? Ann. N.Y. Acad. Sci. 1063, 358–360 (2005).
Rikihisa, Y. Ehrlichia subversion of host innate responses. Curr. Opin. Microbiol. 9, 95–101 (2006).
Rikihisa, Y. Molecular events involved in cellular invasion by Ehrlichia chaffeensis and Anaplasma phagocytophilum. Vet. Parasitol. 167, 155–166 (2010).
Winslow, G. M. & Bitsaktsis, C. Immunity to the ehrlichiae: new tools and recent developments. Curr. Opin. Infect. Dis. 18, 217–221 (2005).
Carlyon, J. A. & Fikrig, E. Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr. Opin. Hematol. 13, 28–33 (2006).
Rikihisa, Y. in Intracellular Niches of Microbes. A Pathogen's Guide Through the Host Cell (eds Schaible, U. & Haas, A.) 301–314 (Wiley-VCH, Weinheim, 2009).
Ewing, S. A. et al. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 32, 368–374 (1995).
Telford, S. R. 3rd et al. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl Acad. Sci. USA 93, 6209–6214 (1996).
Gieg, J., Rikihisa, Y. & Wellman, M. Diagnosis of Ehrlichia ewingii infection by PCR in a puppy from Ohio. Vet. Clin. Pathol. 38, 406–410 (2009).
Buller, R. S. et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 341, 148–155 (1999).
Steiert, J. G. & Gilfoy, F. Infection rates of Amblyomma americanum and Dermacentor variabilis by Ehrlichia chaffeensis and Ehrlichia ewingii in southwest Missouri. Vector Borne Zoonotic Dis. 2, 53–60 (2002).
Anziani, O. S., Ewing, S. A. & Barker, R. W. Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am. J. Vet. Res. 51, 929–931 (1990).
Zhang, L. et al. Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA 300, 2263–2270 (2008).
Krause, P. J. & Wormser, G. P. Nosocomial transmission of human granulocytic anaplasmosis? JAMA 300, 2308–2309 (2008).
Lin, M. & Rikihisa, Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect. Immun. 71, 5324–5331 (2003). The work described in this article shows that E. chaffeensis and A. phagocytophilum lack lipid A and peptidoglycan and that incorporation of host cholesterol is essential for their survival.
Herron, M. J. et al. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science 288, 1653–1656 (2000). The authors show that PSGL1-specific antibody blocks A. phagocytophilum binding and infection of host cells and that dual transfection of non-permissive cells with PSGL1 and sialyl LewisX confers the ability to bind A. phagocytophilum and become infected.
Reneer, D. V. et al. Characterization of a sialic acid- and P-selectin glycoprotein ligand-1-independent adhesin activity in the granulocytotropic bacterium Anaplasma phagocytophilum. Cell. Microbiol. 8, 1972–1984 (2006).
Munderloh, U. G. et al. Infection of endothelial cells with Anaplasma marginale and A. phagocytophilum. Vet. Microbiol. 101, 53–64 (2004).
Carlyon, J. A. et al. Murine neutrophils require α1,3-fucosylation but not PSGL-1 for productive infection with Anaplasma phagocytophilum. Blood 102, 3387–3395 (2003).
Lin, M. & Rikihisa, Y. Obligatory intracellular parasitism by Ehrlichia chaffeensis and Anaplasma phagocytophilum involves caveolae and glycosylphosphatidylinositol-anchored proteins. Cell. Microbiol. 5, 809–820 (2003).
Mott, J., Rikihisa, Y. & Tsunawaki, S. Effects of Anaplasma phagocytophila on NADPH oxidase components in human neutrophils and HL-60 cells. Infect. Immun. 70, 1359–1366 (2002).
Carlyon, J. A., Abdel-Latif, D., Pypaert, M., Lacy, P. & Fikrig, E. Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infect. Immun. 72, 4772–4783 (2004).
IJdo, J. & Mueller, A. C. Neutrophil NADPH oxidase is reduced at the Anaplasma phagocytophilum phagosome. Infect. Immun. 72, 5392–5401 (2004).
Lin, M. & Rikihisa, Y. Degradation of p22phox and inhibition of superoxide generation by Ehrlichia chaffeensis in human monocytes. Cell. Microbiol. 9, 861–874 (2007).
Webster, P., IJdo, J. W., Chicoine, L. M. & Fikrig, E. The agent of Human Granulocytic Ehrlichiosis resides in an endosomal compartment. J. Clin. Invest. 101, 1932–1941 (1998).
Mott, J., Barnewall, R. E. & Rikihisa, Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect. Immun. 67, 1368–1378 (1999).
Niu, H., Yamaguchi, M. & Rikihisa, Y. Subversion of cellular autophagy by Anaplasma phagocytophilum. Cell. Microbiol. 10, 593–605 (2008). This study shows that A. phagocytophilum subverts autophagy to establish itself in an early- autophagosome-like compartment that is segregated from lysosomes and that the induction of autophagy is required for bacterial growth.
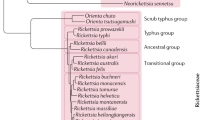
Dunning Hotopp, J. C. et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2, e21 (2006). A detailed analysis of the genomes of three human pathogens, A. phagocytophilum, E. chaffeensis and N. sennetsu.
Omsland, A. et al. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc. Natl Acad. Sci. USA 106, 4430–4434 (2009).
Andersson, S. G. et al. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396, 133–140 (1998).
Stephens, R. S. et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282, 754–759 (1998).
Seshadri, R. et al. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc. Natl Acad. Sci. USA 100, 5455–5460 (2003).
Lin, M., Zhang, C., Gibson, K. & Rikihisa, Y. Analysis of complete genome sequence of Neorickettsia risticii: causative agent of Potomac horse fever. Nucleic Acid Res. 37, 6076–6079 (2009).
Foster, J. et al. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 3, e121 (2005).
Wu, M. et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2, e69 (2004).
Brayton, K. A. et al. Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc. Natl Acad. Sci. USA 102, 844–849 (2005).
Collins, N. E. et al. The genome of the heartwater agent Ehrlichia ruminantium contains multiple tandem repeats of actively variable copy number. Proc. Natl Acad. Sci. USA 102, 838–843 (2005).
Mavromatis, K. et al. The genome of the obligately intracellular bacterium Ehrlichia canis reveals themes of complex membrane structure and immune evasion strategies. J. Bacteriol. 188, 4015–4023 (2006).
Ogata, H. et al. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 293, 2093–2098 (2001).
Felsheim, R. F. et al. Transformation of Anaplasma phagocytophilum. BMC Biotechnol. 6, 42 (2006). This article describes the first transformation of A. phagocytophilum with the Himar transposase system.
Ge, Y. & Rikihisa, Y. Identification of novel surface proteins of Anaplasma phagocytophilum by affinity purification and proteomics. J. Bacteriol. 189, 7819–7828 (2007).
Ge, Y. & Rikihisa, Y. Surface-exposed proteins of Ehrlichia chaffeensis. Infect. Immun. 75, 3833–3841 (2007).
Unver, A. et al. Western blot analysis of sera reactive to human monocytic ehrlichiosis and human granulocytic ehrlichiosis agents. J. Clin. Microbiol. 39, 3982–3986 (2001).
Unver, A. et al. Western and dot blotting analyses of Ehrlichia chaffeensis indirect fluorescent-antibody assay-positive and -negative human sera by using native and recombinant E. chaffeensis and E. canis antigens. J. Clin. Microbiol. 37, 3888–3895 (1999).
Zhi, N. et al. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J. Clin. Microbiol. 36, 1666–1673 (1998).
Zhi, N., Rikihisa, Y., Kim, H. Y., Wormser, G. P. & Horowitz, H. W. Comparison of major antigenic proteins of six strains of the human granulocytic ehrlichiosis agent by Western immunoblot analysis. J. Clin. Microbiol. 35, 2606–2611 (1997).
IJdo, J. W., Wu, C., Magnarelli, L. A. & Fikrig, E. Serodiagnosis of human granulocytic ehrlichiosis by a recombinant HGE-44-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37, 3540–3544 (1999).
Lin, Q., Rikihisa, Y., Ohashi, N. & Zhi, N. Mechanisms of variable p44 expression by Anaplasma phagocytophilum. Infect. Immun. 71, 5650–5661 (2003).
Lin, Q. et al. Analysis of sequences and loci of p44 homologs expressed by Anaplasma phagocytophila in acutely infected patients. J. Clin. Microbiol. 40, 2981–2988 (2002).
Zhi, N., Ohashi, N. & Rikihisa, Y. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J. Biol. Chem. 274, 17828–17836 (1999). This is the first study to show that there are many expressible p44 genes with the signature central hypervariable region in the A. phagocytophilum genome.
Barbet, A. F., Lundgren, A., Yi, J., Rurangirwa, F. R. & Palmer, G. H. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect. Immun. 68, 6133–6138 (2000). The first paper about the msp2 expression locus, proposing the segmental gene conversion mechanism of expression.
Barbet, A. F. et al. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect. Immun. 71, 1706–1718 (2003).
Lin, Q. & Rikihisa, Y. Establishment of cloned Anaplasma phagocytophilum and analysis of p44 gene conversion within an infected horse and infected SCID mice. Infect. Immun. 73, 5106–5114 (2005).
Lin, Q., Zhang, C. & Rikihisa, Y. Analysis of involvement of the RecF pathway in p44 recombination in Anaplasma phagocytophilum and in Escherichia coli by using a plasmid carrying the p44 expression and p44 donor loci. Infect. Immun. 74, 2052–2062 (2006). A description of RecF-dependent p44 recombination, showing a half-crossover structure in A. phagocytophilum and using a double-origin plasmid carrying p44ES and a donor p44 locus in RecF-active and RecF-inactive mutant strains of E. coli.
Wang, X. et al. Rapid sequential changeover of expressed p44 genes during the acute phase of Anaplasma phagocytophilum infection in horses. Infect. Immun. 72, 6852–6859 (2004).
Granquist, E. G. et al. Variant-specific and diminishing immune responses towards the highly variable MSP2(P44) outer membrane protein of Anaplasma phagocytophilum during persistent infection in lambs. Vet. Immunol. Immunopathol. 133, 117–124 (2009).
Granquist, E. G., Stuen, S., Lundgren, A. M., Braten, M. & Barbet, A. F. Outer membrane protein sequence variation in lambs experimentally infected with Anaplasma phagocytophilum. Infect. Immun. 76, 120–126 (2008).
Ladbury, G. A. et al. Dynamic transmission of numerous Anaplasma phagocytophilum genotypes among lambs in an infected sheep flock in an area of anaplasmosis endemicity. J. Clin. Microbiol. 46, 1686–1691 (2008).
Sarkar, M. et al. Anaplasma phagocytophilum MSP2(P44)-18 predominates and is modified into multiple isoforms in human myeloid cells. Infect. Immun. 76, 2090–2098 (2008).
Troese, M. J. et al. Differential expression and glycosylation of Anaplasma phagocytophilum major surface protein 2 paralogs during cultivation in sialyl Lewis x-deficient host cells. Infect. Immun. 77, 1746–1756 (2009).
Scorpio, D. G., Caspersen, K., Ogata, H., Park, J. & Dumler, J. S. Restricted changes in major surface protein-2 (msp2) transcription after prolonged in vitro passage of Anaplasma phagocytophilum. BMC Microbiol. 4, 1 (2004).
Zhi, N. et al. Transcript heterogeneity of the p44 multigene family in a human granulocytic ehrlichiosis agent transmitted by ticks. Infect. Immun. 70, 1175–1184 (2002).
Felek, S., Telford, S. 3rd, Falco, R. C. & Rikihisa, Y. Sequence analysis of p44 homologs expressed by Anaplasma phagocytophilum in infected ticks feeding on naive hosts and in mice infected by tick attachment. Infect. Immun. 72, 659–666 (2004).
Barbet, A. F. et al. Structure of the expression site reveals global diversity in MSP2 (P44) variants in Anaplasma phagocytophilum. Infect. Immun. 74, 6429–6437 (2006).
Wang, X., Cheng, Z., Zhang, C., Kikuchi, T. & Rikihisa, Y. Anaplasma phagocytophilum p44 mRNA expression is differentially regulated in mammalian and tick host cells: involvement of the DNA binding protein ApxR. J. Bacteriol. 189, 8651–8659 (2007).
Wang, X., Kikuchi, T. & Rikihisa, Y. Proteomic identification of a novel Anaplasma phagocytophilum DNA binding protein that regulates a putative transcription factor. J. Bacteriol. 189, 4880–4886 (2007). This study identifies a novel transcription factor in A. phagocytophilum by proteomics, followed by DNA footprint analysis and a lacZ reporter assay.
Nelson, C. M. et al. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC Genomics 9, 364 (2008). The authors describe for the first time the whole-genome transcriptome of A. phagocytophilum in human cell and tick cell culture.
Ohashi, N., Rikihisa, Y. & Unver, A. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 69, 2083–2091 (2001).
Zhang, C., Xiong, Q., Kikuchi, T. & Rikihisa, Y. Identification of 19 polymorphic major outer membrane protein genes and their immunogenic peptides in Ehrlichia ewingii for use in a serodiagnostic assay. Clin. Vaccine Immunol. 15, 402–411 (2008).
van Heerden, H., Collins, N. E., Brayton, K. A., Rademeyer, C. & Allsopp, B. A. Characterization of a major outer membrane protein multigene family in Ehrlichia ruminantium. Gene 330, 159–168 (2004).
Miura, K. & Rikihisa, Y. Virulence potential of Ehrlichia chaffeensis strains of distinct genome sequences. Infect. Immun. 75, 3604–3613 (2007).
Cheng, C., Paddock, C. D. & Reddy Ganta, R. Molecular heterogeneity of Ehrlichia chaffeensis isolates determined by sequence analysis of the 28-kilodalton outer membrane protein genes and other regions of the genome. Infect. Immun. 71, 187–195 (2003).
Long, S. W. et al. Antigenic variation of Ehrlichia chaffeensis resulting from differential expression of the 28-kilodalton protein gene family. Infect. Immun. 70, 1824–1831 (2002).
Yu, X. J., McBride, J. W. & Walker, D. H. Genetic diversity of the 28-kilodalton outer membrane protein gene in human isolates of Ehrlichia chaffeensis. J. Clin. Microbiol. 37, 1137–1143 (1999).
Unver, A., Rikihisa, Y., Stich, R. W., Ohashi, N. & Felek, S. The omp-1 major outer membrane multigene family of Ehrlichia chaffeensis is differentially expressed in canine and tick hosts. Infect. Immun. 70, 4701–4704 (2002).
Zhang, J. Z., Guo, H., Winslow, G. M. & Yu, X. J. Expression of members of the 28-kilodalton major outer membrane protein family of Ehrlichia chaffeensis during persistent infection. Infect. Immun. 72, 4336–4343 (2004).
Singu, V., Liu, H., Cheng, C. & Ganta, R. R. Ehrlichia chaffeensis expresses macrophage- and tick cell-specific 28-kilodalton outer membrane proteins. Infect. Immun. 73, 79–87 (2005).
Unver, A. et al. Transcriptional analysis of p30 major outer membrane multigene family of Ehrlichia canis in dogs, ticks, and cell culture at different temperatures. Infect. Immun. 69, 6172–6178 (2001).
Kim, H. Y. & Rikihisa, Y. Characterization of monoclonal antibodies to the 44-kilodalton major outer membrane protein of the human granulocytic ehrlichiosis agent. J. Clin. Microbiol. 36, 3278–3284 (1998).
Wang, X., Kikuchi, T. & Rikihisa, Y. Two monoclonal antibodies with defined epitopes of P44 major surface proteins neutralize Anaplasma phagocytophilum by distinct mechanisms. Infect. Immun. 74, 1873–1882 (2006).
Park, J., Choi, K. S. & Dumler, J. S. Major surface protein 2 of Anaplasma phagocytophilum facilitates adherence to granulocytes. Infect. Immun. 71, 4018–4025 (2003).
Ohashi, N., Zhi, N., Zhang, Y. & Rikihisa, Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66, 132–139 (1998). The first description of the major outer-membrane OMP1–P28 family proteins of E. chaffeensis , whichare encoded by a multigene family.
Li, J. S., Chu, F., Reilly, A. & Winslow, G. M. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J. Immunol. 169, 1419–1425 (2002).
Winslow, G. M. et al. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect. Immun. 68, 2187–2195 (2000).
Kumagai, Y., Huang, H. & Rikihisa, Y. Expression and porin activity of P28 and OMP-1F during intracellular Ehrlichia chaffeensis development. J. Bacteriol. 190, 3597–3605 (2008).
Huang, H., Wang, X., Kikuchi, T., Kumagai, Y. & Rikihisa, Y. Porin activity of Anaplasma phagocytophilum outer membrane fraction and purified P44. J. Bacteriol. 189, 1998–2006 (2007). The first study to show porin activity of the major outer-membrane protein of a rickettsial pathogen.
Gentle, I. E., Burri, L. & Lithgow, T. Molecular architecture and function of the Omp85 family of proteins. Mol. Microbiol. 58, 1216–1225 (2005).
Voulhoux, R., Bos, M. P., Geurtsen, J., Mols, M. & Tommassen, J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299, 262–265 (2003).
Popov, V. L., Yu, X. & Walker, D. H. The 120 kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb. Pathog. 28, 71–80 (2000).
Doyle, C. K., Nethery, K. A., Popov, V. L. & McBride, J. W. Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect. Immun. 74, 711–720 (2006).
Luo, T., Zhang, X., Wakeel, A., Popov, V. L. & McBride, J. W. A variable-length PCR target protein of Ehrlichia chaffeensis contains major species-specific antibody epitopes in acidic serine-rich tandem repeats. Infect. Immun. 76, 1572–1580 (2008).
McBride, J. W., Yu, X. J. & Walker, D. H. A conserved, transcriptionally active p28 multigene locus of Ehrlichia canis. Gene 254, 245–252 (2000).
Yu, X. J., McBride, J. W., Diaz, C. M. & Walker, D. H. Molecular cloning and characterization of the 120-kilodalton protein gene of Ehrlichia canis and application of the recombinant 120-kilodalton protein for serodiagnosis of canine ehrlichiosis. J. Clin. Microbiol. 38, 369–374 (2000).
McBride, J. W., Yu, X. J. & Walker, D. H. Glycosylation of homologous immunodominant proteins of Ehrlichia chaffeensis and Ehrlichia canis. Infect. Immun. 68, 13–18 (2000).
Luo, T., Zhang, X. & McBride, J. W. Major species-specific antibody epitopes of the Ehrlichia chaffeensis p120 and, E. canis p140 orthologs in surface-exposed tandem repeat regions. Clin. Vaccine Immunol. 16, 982–990 (2009).
Huang, H. et al. Proteomic analysis of and immune responses to Ehrlichia chaffeensis lipoproteins. Infect. Immun. 76, 3405–3414 (2008).
Rikihisa, Y. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol. Rev. 4, 286–308 (1991).
Reichard, M. V. et al. Inoculation of white-tailed deer (Odocoileus virginianus) with Ap-V1 or NY-18 strains of Anaplasma phagocytophilum and microscopic demonstration of Ap-V1 In Ixodes scapularis adults that acquired infection from deer as nymphs. Vector Borne Zoonotic Dis. 9, 565–568 (2009).
Woldehiwet, Z. & Scott, G. R. Stages in the development of Cytoecetes phagocytophila, the causative agent of tick-borne fever. J. Comp. Pathol. 92, 469–474 (1982).
Popov, V. L., Chen, S. M., Feng, H. M. & Walker, D. H. Ultrastructural variation of cultured Ehrlichia chaffeensis. J. Med. Microbiol. 43, 411–421 (1995).
Munderloh, U. G. et al. Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J. Clin. Microbiol. 37, 2518–2524 (1999).
Troese, M. J. & Carlyon, J. A. Anaplasma phagocytophilum dense-cored organisms mediate cellular adherence through recognition of human P-selectin glycoprotein ligand 1. Infect. Immun. 77, 4018–4027 (2009).
Zhang, J. Z., Popov, V. L., Gao, S., Walker, D. H. & Yu, X. J. The developmental cycle of Ehrlichia chaffeensis in vertebrate cells. Cell. Microbiol. 9, 610–618 (2007). An analysis of the E. chaffeensis intracellular developmental cycle and the associated differential expression of cell surface antigens.
Ito, S. & Rikihisa, Y. in Rickettsiae and Rickettsial Diseases (eds Burgdorfer, W. & Anacker, R. L.) 213–227 (Academic Press, New York, 1981).
Cheng, Z., Wang, X. & Rikihisa, Y. Regulation of type IV secretion apparatus genes during Ehrlichia chaffeensis intracellular development by a previously unidentified protein. J. Bacteriol. 190, 2096–2105 (2008).
Lai, T. H., Kumagai, Y., Hyodo, M., Hayakawa, Y. & Rikihisa, Y. The Anaplasma phagocytophilum PleC histidine kinase and PleD diguanylate cyclase two-component system and role of cyclic di-GMP in host cell infection. J. Bacteriol. 191, 693–700 (2009). This article describes the PleCD two-component system and the importance of c-di-GMP for A. phagocytophilum infection.
Niu, H., Rikihisa, Y., Yamaguchi, M. & Ohashi, N. Differential expression of VirB9 and VirB6 during the life cycle of Anaplasma phagocytophilum in human leucocytes is associated with differential binding and avoidance of lysosome pathway. Cell. Microbiol. 8, 523–534 (2006).
Heinzen, R. A. & Hackstadt, T. A developmental stage-specific histone H1 homolog of Coxiella burnetii. J. Bacteriol. 178, 5049–5052 (1996).
Hackstadt, T., Baehr, W. & Ying, Y. Chlamydia trachomatis developmentally regulated protein is homologous to eukaryotic histone H1. Proc. Natl Acad. Sci. USA 88, 3937–3941 (1991).
Cheng, Z., Kumagai, Y., Lin, M., Zhang, C. & Rikihisa, Y. Intra-leukocyte expression of two-component systems in Ehrlichia chaffeensis and Anaplasma phagocytophilum and effects of the histidine kinase inhibitor closantel. Cell. Microbiol. 8, 1241–1252 (2006). Analysis of the three pairs of two-component systems, their potential downstream effectors and their requirement for E. chaffeensis infection.
Kumagai, Y., Cheng, Z., Lin, M. & Rikihisa, Y. Biochemical activities of three pairs of Ehrlichia chaffeensis two-component regulatory system proteins involved in inhibition of lysosomal fusion. Infect. Immun. 74, 5014–5022 (2006).
Romling, U. Cyclic di-GMP (c-di-GMP) goes into host cells—c-di-GMP signaling in the obligate intracellular pathogen Anaplasma phagocytophilum. J. Bacteriol. 191, 683–686 (2009).
Alvarez-Marinez, C. E. & Christie, P. J. C. Biological diversities of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 73, 775–808 (2009).
Rikihisa, Y. & Lin, M. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Curr. Opin. Microbiol. 13, 59–66 (2010).
Bao, W. et al. Four VirB6 paralogs and VirB9 are expressed and interact in Ehrlichia chaffeensis-containing vacuoles. J. Bacteriol. 191, 278–286 (2009).
Lin, M., den Dulk-Ras, A., Hooykaas, P. J. & Rikihisa, Y. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell. Microbiol. 9, 2644–2657 (2007). This study shows that A. phagocytophilum AnkA can be delivered into host cells by a T4SS and binds to ABI1 to activate the tyrosine kinase ABL1and that ABL1 is required for A. phagocytophilum infection.
IJdo, J., Carlson, A. C. & Kennedy, E. L. Anaplasma phagocytophilum AnkA is tyrosine-phosphorylated at EPIYA motifs and recruits SHP-1 during early infection. Cell. Microbiol. 9, 1284–1296 (2007).
Park, J., Kim, K. J., Choi, K. S., Grab, D. J. & Dumler, J. S. Anaplasma phagocytophilum AnkA binds to granulocyte DNA and nuclear proteins. Cell. Microbiol. 6, 743–751 (2004).
Garcia-Garcia, J. C., Rennoll-Bankert, K. E., Pelly, S., Milstone, A. M. & Dumler, J. S. Silencing of host cell CYBB gene expression by the nuclear effector AnkA of the intracellular pathogen Anaplasma phagocytophilum. Infect. Immun. 77, 2385–2391 (2009).
Garcia-Garcia, J. C., Barat, N. C., Trembley, S. J. & Dumler, J. S. Epigenetic silencing of host cell defense genes enhances intracellular survival of the rickettsial pathogen Anaplasma phagocytophilum. PLoS Path. 5, e1000488 (2009). The authors show that host chromatin modifications by histone deacetylase 1 are linked to the downregulation of host defence genes during A. phagocytophilum infection.
Zhu, B. et al. Nuclear translocated Ehrlichia chaffeensis ankyrin protein interacts with a specific adenine-rich motif of host promoter and intronic Alu elements. Infect. Immun. 77, 4243–4255 (2009).
Pan, X., Luhrmann, A., Satoh, A., Laskowski-Arce, M. A. & Roy., C. R. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 320, 1651–1654 (2008).
Wakeel, A., Kuriakose, J. A. & McBride, J. W. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect. Immun. 77, 1734–1745 (2009).
Yoshiie, K., Kim, H. Y., Mott, J. & Rikihisa, Y. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect. Immun. 68, 1125–1133 (2000). The first demonstration that A. phagocytophilum blocks the spontaneous apoptosis of human neutrophils.
Ge, Y., Yoshiie, K., Kuribayashi, F., Lin, M. & Rikihisa, Y. Anaplasma phagocytophilum inhibits human neutrophil apoptosis via upregulation of bfl-1, maintenance of mitochondrial membrane potential and prevention of caspase 3 activation. Cell. Microbiol. 7, 29–38 (2005).
Scaife, H., Woldehiwet, Z., Hart, C. A. & Edwards, S. W. Anaplasma phagocytophilum reduces neutrophil apoptosis in vivo. Infect. Immun. 71, 1995–2001 (2003).
Borjesson, D. L. et al. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J. Immunol. 174, 6364–6372 (2005). This study shows that A. phagocytophilum fails to induce apoptotic differentiation of human neutrophils in cell culture by global microarray analysis.
Choi, K. S., Park, J. T. & Dumler, J. S. Anaplasma phagocytophilum delay of neutrophil apoptosis through the p38 mitogen-activated protein kinase signal pathway. Infect. Immun. 73, 8209–8218 (2005).
Lee, H. C. & Goodman, J. L. Anaplasma phagocytophilum causes global induction of antiapoptosis in human neutrophils. Genomics 88, 496–503 (2006).
Ge, Y. & Rikihisa, Y. Anaplasma phagocytophilum delays spontaneous human neutrophil apoptosis by modulation of multiple apoptotic pathways. Cell. Microbiol. 8, 1406–1416 (2006).
Pedra, J. H., Sukumaran, B., Carlyon, J. A., Berliner, N. & Fikrig, E. Modulation of NB4 promyelocytic leukemic cell machinery by Anaplasma phagocytophilum. Genomics 86, 365–377 (2005).
Zhang, J. Z., Sinha, M., Luxon, B. A. & Yu, X. J. Survival strategy of obligately intracellular Ehrlichia chaffeensis: novel modulation of immune response and host cell cycles. Infect. Immun. 72, 498–507 (2004).
Niu, H., Kozjak-Pavlovic, V., Rudel, T. & Rikihisa, Y. Anaplasma phagocytophilum Ats-1 is Imported into host cell mitochondria. PLoS Path. 6, e1000774 (2010). This work finds that A. phagocytophilum Ats1 is translocated into the mitochondrial matrix of host cells and inhibits apoptosis.
Amano, A., Nakagawa, I. & Yoshimori, T. Autophagy in innate immunity against intracellular bacteria. J. Biochem. 140, 161–166 (2006).
Ling, Y. M. et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J. Exp.Med. 203, 2063–2071 (2006).
Schmid, D., Dengjel, J., Schoor, O., Stevanovic, S. & Munz, C. Autophagy in innate and adaptive immunity against intracellular pathogens. J. Mol. Med. 84, 194–202 (2006).
Xiong, Q., Lin, M. & Rikihisa, Y. Cholesterol-dependent Anaplasma phagocytophilum exploits the low-density lipoprotein uptake pathway. PLoS Path. 5, e1000329 (2009). This article shows that A. phagocytophilum acquires cholesterol from the host low-density-lipoprotein uptake pathway by upregulating the low-density-lipoprotein receptor and increasing host cell cholesterol homeostasis.
Berry, D. S. et al. Ehrlichial meningitis with cerebrospinal fluid morulae. Pediatr. Infect. Dis. J. 18, 552–555 (1999).
Dunn, B. E. et al. Identification of Ehrlichia chaffeensis morulae in cerebrospinal fluid mononuclear cells. J. Clin. Microbiol. 30, 2207–2210 (1992).
Ratnasamy, N., Everett, E. D., Roland, W. E., McDonald, G. & Caldwell, C. W. Central nervous system manifestations of human ehrlichiosis. Clin. Infect. Dis. 23, 314–319 (1996).
Acknowledgements
I thank all former and current members of my laboratory for their scientific contributions, T. Vojt for preparing the figures, V. L. Popov for the electron micrographs and K. Hayes-Ozello for editorial assistance. I also acknowledge grants from the US National Institutes of Health.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
DATABASES
FURTHER INFORMATION
Supplementary information
41579_2010_BFnrmicro2318_MOESM1_ESM.pdf
Supplementary information S1 | Selected surface proteins from Anaplasma phagocytophilum and Ehrlichia chaffeensis (PDF 267 kb)
Glossary
- Caveola
-
A specialized lipid raft region of the plasma membrane that contains the protein caveolin and forms flask-shaped, cholesterol-rich invaginations of the membrane.
- Autophagosome
-
An intracytoplasmic, membrane-bound vacuole containing elements of a cell's own cytoplasm and membrane proteins that are distinct from phagosomes or other intracellular vesicles. It usually fuses with a lysosome.
- Type IV secretion system
-
A multiprotein complex that mediates the translocation of macromolecules (that is, proteins, DNA or DNA–protein complexes) across the bacterial cell envelope into the extracellular medium or directly into recipient cells.
- Himar transposase system
-
A DNA insertion system using Himar1, which belongs to the mariner family of transposable elements. Himar1 requires no host-specific factors for transposition and has been frequently used as a prokaryotic genetic tool.
- Alternative σ-factor
-
A σ-factor that is produced under specific conditions, allowing the RNA polymerase to transcribe a different set of genes than the housekeeping σ factor, σ70, allows.
- Alu elements
-
Short interspersed DNA elements that are abundant in the human genome.
- Apoptosis
-
Programmed cell death. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis usually confers advantages during an organism's life cycle.
- Autophagy
-
A catabolic process involving the degradation of a cell's own components through the lysosomal machinery. It is a tightly regulated process that plays a normal part in cell growth, development and homeostasis, helping to maintain a balance between the synthesis, degradation and subsequent recycling of cellular products.
Rights and permissions
About this article
Cite this article
Rikihisa, Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Microbiol 8, 328–339 (2010). https://doi.org/10.1038/nrmicro2318
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2318
This article is cited by
-
Anaplasma phagocytophilum Ats-1 enhances exosome secretion through Syntenin-1
BMC Microbiology (2023)
-
Genome-Wide Subtraction Analysis and Reverse Vaccinology to Detect Novel Drug Targets and Potential Vaccine Candidates Against Ehrlichia chaffeensis
Applied Biochemistry and Biotechnology (2023)
-
The obligate intracellular bacterium Orientia tsutsugamushi differentiates into a developmentally distinct extracellular state
Nature Communications (2022)
-
Diversity unearthed by the estimated molecular phylogeny and ecologically quantitative characteristics of uncultured Ehrlichia bacteria in Haemaphysalis ticks, Japan
Scientific Reports (2021)
-
Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle
Nature Reviews Microbiology (2021)