Key Points
-
Direct-acting antiviral agents targeting the viral NS3/4A protease, NS5B polymerase and NS5A protein have been developed
-
Combinations of antiviral agents with different mechanism of action lead to high rates of sustained virologic response without the need for PEG-IFN
-
Classes of direct-acting antiviral agents have different antiviral attributes such as barrier to resistance and genotypic coverage, and different clinical attributes such as adverse effects, dosing, and drug–drug interactions
-
The importance of resistance-associated variants present at baseline or after a course of unsuccessful therapy differs by antiviral class and the genotype subtype
Abstract
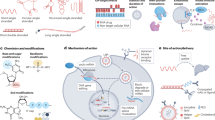
The treatment of HCV infection has evolved at an extremely rapid pace over the past few years. The development of direct-acting antiviral agents, which potently inhibit different stages in the viral life cycle, has led to the replacement of interferon with well-tolerated oral therapies with cure rates of >90% in most patient populations. Understanding the mechanisms of action of the various agents as well as related issues, including the molecular basis for resistance, helps to guide drug development and clinical use. In this Review, we provide a mechanistic description of NS3/4A protease inhibitors, nucleotide and non-nucleotide inhibitors of the NS5B viral polymerase and inhibitors of the NS5A protein, followed by a summary of clinical data from studies of each drug class alone and in combination. Remaining challenges in drug development efforts are also discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mohd Hanafiah, K., Groeger, J., Flaxman, A. D. & Wiersma, S. T. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57, 1333–1342 (2013).
Bialek, S. R. & Terrault, N. A. The changing epidemiology and natural history of hepatitis C virus infection. Clin. Liver Dis. 10, 697–715 (2006).
Rupp, D. & Bartenschlager, R. Targets for antiviral therapy of hepatitis C. Semin. Liver Dis. 34, 9–21 (2014).
Hézode, C. et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) — NCT01514890. J. Hepatol. 59, 434–441 (2013).
Afdhal, N. et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N. Engl. J. Med. 370, 1483–1493 (2014).
Feld, J. J. et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N. Engl. J. Med. 370, 1594–1603 (2014).
Bartenschlager, R., Lohmann, V. & Penin, F. The molecular and structural basis of advanced antiviral therapy for hepatitis C virus infection. Nat. Rev. Microbiol. 11, 482–496 (2013).
Lange, C. M., Jacobson, I. M., Rice, C. M. & Zeuzem, S. Emerging therapies for the treatment of hepatitis C. EMBO Mol. Med. 6, 4–15 (2014).
Scheel, T. K. & Rice, C. M. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat. Med. 19, 837–849 (2013).
Lohmann, V. et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285, 110–113 (1999).
Zhong, J. et al. Robust hepatitis C virus infection in vitro. Proc. Natl Acad. Sci. USA 102, 9294–9299 (2005).
Lindenbach, B. D. et al. Complete replication of hepatitis C virus in cell culture. Science 309, 623–626 (2005).
Wakita, T. et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11, 791–796 (2005).
Love, R. A. et al. The crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding site. Cell 87, 331–342 (1996).
Yao, N., Reichert, P., Taremi, S. S., Prosise, W. W. & Weber, P. C. Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease-helicase. Structure 7, 1353–1363 (1999).
Lamarre, D. et al. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426, 186–189 (2003).
Schiering, N. et al. A macrocyclic HCV NS3/4A protease inhibitor interacts with protease and helicase residues in the complex with its full-length target. Proc. Natl Acad. Sci. USA 108, 21052–21056 (2011).
Kwong, A. D., Kauffman, R. S., Hurter, P. & Mueller, P. Discovery and development of telaprevir: an NS3-4A protease inhibitor for treating genotype 1 chronic hepatitis C virus. Nat. Biotechnol. 29, 993–1003 (2011).
Hazuda, D. J., Burroughs, M., Howe, A. Y., Wahl, J. & Venkatraman, S. Development of boceprevir: a first-in-class direct antiviral treatment for chronic hepatitis C infection. Ann. NY Acad. Sci. 1291, 69–76 (2013).
Romano, K. P. et al. The molecular basis of drug resistance against hepatitis C virus NS3/4A protease inhibitors. PLoS Pathog. 8, e1002832 (2012).
Romano, K. P., Ali, A., Royer, W. E. & Schiffer, C. A. Drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding. Proc. Natl Acad. Sci. USA 107, 20986–20991 (2010).
Romano, K. P. et al. Molecular mechanisms of viral and host cell substrate recognition by hepatitis C virus NS3/4A protease. J. Virol. 85, 6106–6116 (2011).
Pawlotsky, J. M. Hepatitis C treatment: the data flood goes on — an update from the liver meeting 2014. Gastroenterology 148, 468–479 (2015).
Summa, V. et al. MK-5172, a selective inhibitor of hepatitis C virus NS3/4a protease with broad activity across genotypes and resistant variants. Antimicrob. Agents Chemother. 56, 4161–4167 (2012).
Liang, Y. et al. Antiviral suppression versus restoration of RIG-I signaling by hepatitis C protease and polymerase inhibitors. Gastroenterology 135, 1710–1718.e2 (2008).
Sofia, M. J., Chang, W., Furman, P. A., Mosley, R. T. & Ross, B. S. Nucleoside, nucleotide, and non-nucleoside inhibitors of hepatitis C virus NS5B RNA-dependent RNA-polymerase. J. Med. Chem. 55, 2481–2531 (2012).
Lesburg, C. A. et al. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat. Struct. Biol. 6, 937–943 (1999).
Bressanelli, S. et al. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Proc. Natl Acad. Sci. USA 96, 13034–13039 (1999).
Biswal, B. K. et al. Crystal structures of the RNA-dependent RNA polymerase genotype 2a of hepatitis C virus reveal two conformations and suggest mechanisms of inhibition by non-nucleoside inhibitors. J. Biol. Chem. 280, 18202–18210 (2005).
Appleby, T. C. et al. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 347, 771–775 (2015).
Di Marco, S. et al. Interdomain communication in hepatitis C virus polymerase abolished by small molecule inhibitors bound to a novel allosteric site. J. Biol. Chem. 280, 29765–29770 (2005).
Rigat, K. L. et al. Mechanism of inhibition for BMS-791325, a novel non-nucleoside inhibitor of hepatitis C virus NS5B polymerase. J. Biol. Chem. 289, 33456–33468 (2014).
Kukolj, G. et al. Binding site characterization and resistance to a class of non-nucleoside inhibitors of the hepatitis C virus NS5B polymerase. J. Biol. Chem. 280, 39260–39267 (2005).
Biswal, B. K. et al. Non-nucleoside inhibitors binding to hepatitis C virus NS5B polymerase reveal a novel mechanism of inhibition. J. Mol. Biol. 361, 33–45 (2006).
Dvory-Sobol, H. et al. Clinical and in vitro resistance to GS-9669, a thumb site II nonnucleoside inhibitor of the hepatitis C virus NS5B polymerase. Antimicrob. Agents Chemother. 58, 6599–6606 (2014).
Kati, W. et al. In vitro activity and resistance profile of dasabuvir, a nonnucleoside hepatitis C virus polymerase inhibitor. Antimicrob. Agents Chemother. 59, 1505–1511 (2015).
Dutartre, H., Bussetta, C., Boretto, J. & Canard, B. General catalytic deficiency of hepatitis C virus RNA polymerase with an S282T mutation and mutually exclusive resistance towards 2′-modified nucleotide analogues. Antimicrob. Agents Chemother. 50, 4161–4169 (2006).
Svarovskaia, E. S. et al. Infrequent development of resistance in genotype 1–6 hepatitis C virus-infected subjects treated with sofosbuvir in phase 2 and 3 clinical trials. Clin. Infect. Dis. 59, 1666–1674 (2014).
Tong, X. et al. In vivo emergence of a novel mutant L159F/L320F in the NS5B polymerase confers low-level resistance to the HCV polymerase inhibitors mericitabine and sofosbuvir. J. Infect. Dis. 209, 668–675 (2014).
Nguyen, L. T. Naturally occurring HCV NS5A/B inhibitor resistance-associated mutations to direct-acting antivirals. Antivir. Ther. http://dx.doi.org/10.3851/IMP3025 (2016).
Donaldson, E. F., Harrington, P. R., O'Rear, J. J. & Naeger, L. K. Clinical evidence and bioinformatics characterization of potential hepatitis C virus resistance pathways for sofosbuvir. Hepatology 61, 56–65 (2015).
Ross-Thriepland, D. & Harris, M. Hepatitis C virus NS5A: enigmatic but still promiscuous 10 years on! J. Gen. Virol. 96, 727–738 (2015).
Tellinghuisen, T. L., Marcotrigiano, J. & Rice, C. M. Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase. Nature 435, 374–379 (2005).
Love, R. A., Brodsky, O., Hickey, M. J., Wells, P. A. & Cronin, C. N. Crystal structure of a novel dimeric form of NS5A domain I protein from hepatitis C virus. J. Virol. 83, 4395–4403 (2009).
Lambert, S. M. et al. The crystal structure of NS5A domain 1 from genotype 1a reveals new clues to the mechanism of action for dimeric HCV inhibitors. Protein Sci. 23, 723–734 (2014).
Verdegem, D. et al. Domain 3 of NS5A protein from the hepatitis C virus has intrinsic α-helical propensity and is a substrate of cyclophilin A. J. Biol. Chem. 286, 20441–20454 (2011).
Hanoulle, X., Badillo, A., Verdegem, D., Penin, F. & Lippens, G. The domain 2 of the HCV NS5A protein is intrinsically unstructured. Protein Pept. Lett. 17, 1012–1018 (2010).
Belema, M. & Meanwell, N. A. Discovery of daclatasvir, a pan-genotypic hepatitis C virus NS5A replication complex inhibitor with potent clinical effect. J. Med. Chem. 57, 5057–5071 (2014).
Feld, J. J. Interferon-free strategies with a nucleoside/nucleotide analogue. Semin. Liver Dis. 34, 37–46 (2014).
Barakat, K. H. et al. A refined model of the HCV NS5A protein bound to daclatasvir explains drug-resistant mutations and activity against divergent genotypes. J. Chem. Inf. Model. 55, 362–373 (2015).
Berger, C. et al. Daclatasvir-like inhibitors of NS5A block early biogenesis of hepatitis C virus-induced membranous replication factories, independent of RNA replication. Gastroenterology 147, 1094–1105.e25 (2014).
Nettles, J. H. et al. Asymmetric binding to NS5A by daclatasvir (BMS-790052) and analogs suggests two novel modes of HCV inhibition. J. Med. Chem. 57, 10031–10043 (2014).
Ascher, D. B. et al. Potent hepatitis C inhibitors bind directly to NS5A and reduce its affinity for RNA. Sci. Rep. 4, 4765 (2014).
Sarrazin, C. et al. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 132, 1767–1777 (2007).
Hézode, C. et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N. Engl. J. Med. 360, 1839–1850 (2009).
Feld, J. J. Is there a role for ribavirin in the era of hepatitis C virus direct-acting antivirals? Gastroenterology 142, 1356–1359 (2012).
Jacobson, I. M. et al. A practical guide for the use of boceprevir and telaprevir for the treatment of hepatitis C. J. Viral Hepat. 19 (Suppl. 2), 1–26 (2012).
Zeuzem, S. et al. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology 146, 430–441.e6 (2014).
Dierynck, I. et al. Deep-sequencing analysis of the gene encoding the hepatitis C virus nonstructural 3-4A protease confirms a low prevalence of telaprevir-resistant variants at baseline and the end of the REALIZE study. J. Infect. Dis. 210, 1871–1880 (2014).
Nguyen, L. T. et al. Baseline prevalence and emergence of protease inhibitor resistance mutations following treatment in chronic HCV genotype-1-infected individuals. Antivir. Ther. 20, 865–869 (2014).
Manns, M. et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 384, 414–426 (2014).
Jacobson, I. M. et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 384, 403–413 (2014).
Lok, A. S. et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 366, 216–224 (2012).
Chayama, K. et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology 55, 742–748 (2012).
Ribeiro, R. M. et al. Quantifying the diversification of hepatitis C virus (HCV) during primary infection: estimates of the in vivo mutation rate. PLoS Pathog. 8, e1002881 (2012).
Powdrill, M. H. et al. Contribution of a mutational bias in hepatitis C virus replication to the genetic barrier in the development of drug resistance. Proc. Natl Acad. Sci. USA 108, 20509–20513 (2011).
Chayama, K. & Hayes, C. N. HCV drug resistance challenges in Japan: the role of pre-existing variants and emerging resistant strains in direct acting antiviral therapy. Viruses 7, 5328–5342 (2015).
Lawitz, E. et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 384, 1756–1765 (2014).
Kwo, P. et al. A phase 3, randomised, open-label study to evaluate the efficacy and safety of 8 and 12 weeks of simeprevir (SMV) plus sofosbuvir (SOF) in treatment-naïve and -experienced patients with chronic HCV genotype 1 infection without cirrhosis: optimist-1[abstract LP14]. J. Hepatol. 62, S270 (2015).
Lawitz, E. et al. A phase 3, open-label, single-arm study to evaluate the efficacy and safety of 12 weeks of simeprevir (SMV) plus sofosbuvir (SOF) in treatment-naïve or -experienced patients with chronic HCV genotype 1 infection and cirrhosis: optimist-2 [abstract LP04]. J. Hepatol. 62, S264–S265 (2015).
AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 62, 932–954 (2015).
Poordad, F. et al. Fixed-dose combination therapy with daclatasvir, asunaprevir, and beclabuvir for noncirrhotic patients with HCV genotype 1 infection. JAMA 313, 1728–1735 (2015).
Lawitz, E. et al. Once daily sofosbuvir/ledipasvir fixed dose combination with or without ribavirin resulted in ≥95% sustained virologic response in patients with HCV genotype 1, including patients with cirrhosis: the LONESTAR trial [oral presentation]. Hepatology 58, 86A (2013).
Gentile, I. et al. ABT-450: a novel protease inhibitor for the treatment of hepatitis C virus infection. Curr. Med. Chem. 21, 3261–3270 (2014).
Ferenci, P. et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N. Engl. J. Med. 370, 1983–1992 (2014).
Feld, J. J. et al. TURQUOISE-III: safety and efficacy of 12-week ribavirin-free treatment for patients with HCV genotype 1b and cirrhosis [poster presentation P226]. J. Viral Hepat. 22 (Suppl. 2), 134–135 (2015).
Poordad, F. et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N. Engl. J. Med. 370, 1973–1982 (2014).
Pol, S. et al. Interferon-free regimens of ombitasvir and ABT-450/r with or without ribavirin in patients with HCV genotype 4 infection: PEARL-I study results [abstract 1928]. Hepatology 60 (Suppl. 1), 1128A (2014).
Forns, X. et al. C-SALVAGE: grazoprevir (GZR; MK-5172), elbasvir (EBR; MK-8742) and ribavirin (RBV) for chronic HCV-genotype 1 (GT1) infection after failure of direct-acting antiviral (DAA) therapy [abstract O001]. J. Hepatol. 62, S190 (2015).
Zeuzem, S. et al. The phase 3 C-EDGE treatment-naïve (TN) study of a 12-week oral regimen of grazoprevir (GZR, MK-5172)/elbasvir (EBR, MK-8742) in patients with chronic HCV genotype (GT) 1, 4, or 6 infection [abstract G07]. J. Hepatol. 62, S213 (2015).
Krishnan, P. et al. Long-term follow-up of treatment-emergent resistance-associated variants in NS3, NS5A and NS5B with paritaprevir/r-, ombitasvir- and dasabuvir-based regimens [abstract O057]. J. Hepatol. 62, S220 (2015).
Kohli, A. et al. Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study. Lancet 385, 1107–1113 (2015).
McKenzie, R. et al. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N. Engl. J. Med. 333, 1099–1105 (1995).
Dyson, J. K. et al. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J. Hepatol. 64, 234–238 (2016).
Welker, M. W. et al. Lactic acidosis in patients with hepatitis C virus cirrhosis and combined ribavirin/sofosbuvir treatment. J. Hepatol. 64, 790–799 (2016).
Renet, S. et al. Extreme bradycardia after first doses of sofosbuvir and daclatasvir in patients receiving amiodarone: 2 cases including a rechallenge. Gastroenterology 149, 1378–1380.e1 (2015).
Lawitz, E. et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N. Engl. J. Med. 368, 1878–1887 (2013).
Jacobson, I. M. et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N. Engl. J. Med. 368, 1867–1877 (2013).
Gane, E. J. et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N. Engl. J. Med. 368, 34–44 (2013).
Zeuzem, S. et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N. Engl. J. Med. 370, 1993–2001 (2014).
Lawitz, E. et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet 383, 515–523 (2014).
Foster, G. R. et al. Sofosbuvir + peginterferon/ribavirin for 12 weeks versus sofosbuvir + ribavirin for 16 or 24 weeks in genotype 3 HCV infected patients and treatment-experienced cirrhotic patients with genotype 2 HCV: the Boson Study [abstract LO5]. J. Hepatol. 62, S259–S260 (2015).
Sulkowski, M. S. et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N. Engl. J. Med. 370, 211–221 (2014).
Afdhal, N. et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 370, 1889–1898 (2014).
Kowdley, K. V. et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N. Engl. J. Med. 370, 1879–1888 (2014).
Brown, R. S. et al. Implications of baseline HCVRNA level and intrapatient viral load variability on OBV/PTV/R + DSV 12-week treatment outcomes[abstract LP39]. J. Hepatol. 62, S283 (2015).
O'Brien, T. R., Feld, J. J., Kottilil, S. & Pfeiffer, R. M. No scientific basis to restrict 8 weeks of treatment with ledipasvir/sofosbuvir to patients with hepatitis C virus RNA <6,000,000 IU/mL. Hepatology 63, 28–30 (2016).
Bourliere, M. et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect. Dis. 15, 397–404 (2015).
Charlton, M. et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 149, 649–659 (2015).
Gane, E. J. et al. Safety and efficacy of short-duration treatment with GS-9857 combined with sofosbuvir/GS-5816 in treatment-naïve and DAA-experienced genotype 1 patients with and without cirrhosis [abstract LP03]. J. Hepatol. 62, S264 (2015).
Poordad, F. et al. C-swift: grazoprevir/elbasvir + sofosbuvir in cirrhotic and noncirrhotic, treatment-naïve patients with hepatitis C virus genotype 1 infection, for durations of 4, 6 or 8 weeks and genotype 3 infection for durations of 8 or 12 weeks [abstract O006]. J. Hepatol. 62, S192–S193 (2015).
Lawitz, E. et al. Retreatment of patients who failed 8 or 12 weeks of ledipasvir/sofosbuvir-based regimens with ledipasvir/sofosbuvir for 24 weeks [abstract O005]. J. Hepatol. 62, S192 (2015).
Manns, M. et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet 384, 1597–1605 (2014).
Sarrazin, C. et al. The prevalence and the effect of HCV NS5A resistance associated variants in subjects with compensated cirrhosis treated with ledipasvir/sofosbuvir ± RBV[abstract P0773]. J. Hepatol. 62, S620 (2015).
Feld, J. J. et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N. Engl. J. Med. 373, 2599–2607 (2015).
Foster, G. R. et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N. Engl. J. Med. 373, 2608–2617 (2015).
Li, Q. et al. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc. Natl Acad. Sci. USA 106, 16410–16415 (2009).
Baugh, J. M., Garcia-Rivera, J. A. & Gallay, P. A. Host-targeting agents in the treatment of hepatitis C: a beginning and an end? Antiviral Res. 100, 555–561 (2013).
He, S. et al. Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci. Transl. Med. 7, 282ra49 (2015).
Roth, D. et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 386, 1537–1545 (2015).
Dore, G. J. & Feld, J. J. Hepatitis C virus therapeutic development: in pursuit of 'perfectovir'. Clin. Infect. Dis. 60, 1829–1836 (2015).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
M.G. has received scientific consulting fees and/or research support from AstraZeneca, Gilead Sciences, GlaxoSmithKline, Medivir, Merck, Microbiotix, Pfizer and Tibotec/Johnson & Johnson, as well as funding from the Canadian Institutes of Health Research. J.J.F. has received scientific consulting fees and/or research support from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and Theravance, as well as grants from Physician Services' Incorporated Foundation, the National Institutes of Health and the Canadian Institutes of Health Research.
Rights and permissions
About this article
Cite this article
Götte, M., Feld, J. Direct-acting antiviral agents for hepatitis C: structural and mechanistic insights. Nat Rev Gastroenterol Hepatol 13, 338–351 (2016). https://doi.org/10.1038/nrgastro.2016.60
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.60
This article is cited by
-
Prevalence, risk factors, treatment uptake and treatment outcome of hepatitis C virus in people who inject drugs at the needle and syringe program in Uppsala, Sweden
Harm Reduction Journal (2023)
-
Compositions of gut microbiota before and shortly after hepatitis C viral eradication by direct antiviral agents
Scientific Reports (2022)
-
Regression of liver fibrosis and hepatocellular carcinoma development after HCV eradication with oral antiviral agents
Scientific Reports (2022)
-
Optimization of AAV vectors to target persistent viral reservoirs
Virology Journal (2021)
-
9-Sulfonyl-9(H)-Purine Derivatives Inhibit HCV Replication Via their Degradation Species
Pharmaceutical Chemistry Journal (2021)