Abstract
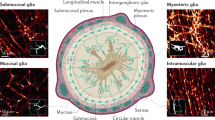
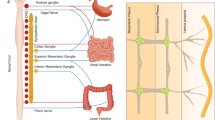
Enteric glia are a unique class of peripheral glial cells within the gastrointestinal tract. Major populations of enteric glia are found in enteric ganglia in the myenteric and submucosal plexuses of the enteric nervous system (ENS); these cells are also found outside of the ENS, within the circular muscle and in the lamina propria of the mucosa. These different populations of cells probably represent unique classes of glial cells with differing functions. In the past few years, enteric glia have been found to be involved in almost every gut function including motility, mucosal secretion and host defence. Subepithelial glia seem to have a trophic and supporting relationship with intestinal epithelial cells, but the necessity of these roles in the maintenance of normal epithelial functions remains to be shown. Likewise, glia within enteric ganglia are activated by synaptic stimulation, suggesting an active role in synaptic transmission, but the precise role of glial activation in normal enteric network activity is unclear. Excitingly, enteric glia can also give rise to new neurons, but seemingly only under limited circumstances. In this Review, we discuss the current body of evidence supporting functional roles of enteric glia and identify key gaps in our understanding of the physiology of these unique cells.
Key Points
-
Unique populations of glial cells reside at multiple levels through the gut wall along the length of the gastrointestinal tract
-
At the level of the mucosa, enteric glia influence epithelial cells and, thus, epithelial barrier function
-
Within enteric ganglia, enteric glia are similar to the astrocytes of the central nervous system, detecting and integrating neural activity
-
Enteric glia have the potential to modulate enteric neurotransmission, but exactly how they influence enteric circuits is unknown
-
Enteric glia have a neurogenic capacity in vitro that seems to be largely suppressed in vivo
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dogiel, A. S. Über den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugetiere [German]. Arch. Anat. Physiol. Leipzig. Anat. Abt. Jg. 1899, 130–158 (1899).
Gabella, G. Fine structure of the myenteric plexus in the guinea-pig ileum. J. Anat. 111, 69–97 (1972).
Gershon, M. D. & Rothman, T. P. Enteric glia. Glia 4, 195–204 (1991).
Hanani, M. et al. Patch-clamp study of neurons and glial cells in isolated myenteric ganglia. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G644–G651 (2000).
Jessen, K. R. & Mirsky, R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 286, 736–737 (1980).
Ferri, G. L. et al. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature 297, 409–410 (1982).
Hoff, S. et al. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J. Comp. Neurol. 509, 356–371 (2008).
Laranjeira, C. & Pachnis, V. Enteric nervous system development: recent progress and future challenges. Auton. Neurosci. 151, 61–69 (2009).
Dulac, C. & Le Douarin, N. M. Phenotypic plasticity of Schwann cells and enteric glial cells in response to the microenvironment. Proc. Natl Acad. Sci. USA 88, 6358–6362 (1991).
Hanani, M. & Reichenbach, A. Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res. 278, 153–160 (1994).
Jessen, K. R. & Mirsky, R. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J. Neurosci. 3, 2206–2218 (1983).
Chen, H. et al. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am. J. Physiol. Cell. Physiol. 292, C497–C507 (2007).
Nasser, Y., Ho, W. & Sharkey, K. A. Distribution of adrenergic receptors in the enteric nervous system of the guinea pig, mouse, and rat. J. Comp. Neurol. 495, 529–553 (2006).
Costagliola, A., Van Nassauw, L., Snyders, D., Adriaensen, D. & Timmermans, J. P. Voltage-gated delayed rectifier K v 1-subunits may serve as distinctive markers for enteroglial cells with different phenotypes in the murine ileum. Neurosci. Lett. 461, 80–84 (2009).
Maudlej, N. & Hanani, M. Modulation of dye coupling among glial cells in the myenteric and submucosal plexuses of the guinea pig. Brain Res. 578, 94–98 (1992).
Savidge, T. C. et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132, 1344–1358 (2007).
Bach-Ngohou, K. et al. Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-12, 14-prostaglandin J2. J. Physiol. 588, 2533–2544 (2010).
Neunlist, M. et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-β1-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G231–G241 (2007).
Van Landeghem, L. et al. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G976–G987 (2011).
Van Landeghem, L. et al. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics 10, 507 (2009).
Aube, A. C. et al. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 55, 630–637 (2006).
Bush, T. G. et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93, 189–201 (1998).
Cornet, A. et al. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc. Natl Acad. Sci. USA 98, 13306–13311 (2001).
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 (2010).
von Boyen, G. B. et al. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 11, 3 (2011).
Bradley, J. S. Jr, Parr, E. J. & Sharkey, K. A. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res. 289, 455–461 (1997).
Joseph, N. M. et al. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Invest. 121, 3398–3411 (2011).
Nasser, Y., Keenan, C. M., Ma, A. C., McCafferty, D. M. & Sharkey, K. A. Expression of a functional metabotropic glutamate receptor 5 on enteric glia is altered in states of inflammation. Glia 55, 859–872 (2007).
Geboes, K. et al. Major histocompatibility class II expression on the small intestinal nervous system in Crohn's disease. Gastroenterology 103, 439–447 (1992).
Nasser, Y. et al. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G912–G927 (2006).
Aikawa, H. & Suzuki, K. Enteric gliopathy in niacin-deficiency induced by CNS glio-toxin. Brain Res. 334, 354–356 (1985).
Aikawa, H. & Suzuki, K. Lesions in the skin, intestine, and central nervous system induced by an antimetabolite of niacin. Am. J. Pathol. 122, 335–342 (1986).
Krum, J. M. Age-dependent susceptibility of CNS glial populations in situ to the antimetabolite 6-aminonicotinamide. Mol. Chem. Neuropathol. 26, 79–94 (1995).
Costantini, T. W. et al. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1308–G1318 (2010).
Vanderwinden, J. M., Timmermans, J. P. & Schiffmann, S. N. Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res. 312, 149–154 (2003).
Fields, R. D. & Ni, Y. Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci. Signal 3, ra73 (2010).
Stys, P. K. The axo-myelinic synapse. Trends Neurosci. 34, 393–400 (2011).
Broadhead, M. J., Bayguinov, P. O., Okamoto, T., Heredia, D. J. & Smith, T. K. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J. Physiol. 590, 335–350 (2012).
Garrido, R., Segura, B., Zhang, W. & Mulholland, M. Presence of functionally active protease-activated receptors 1 and 2 in myenteric glia. J. Neurochem. 83, 556–564 (2002).
Gomes, P. et al. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroent. Motil. 21, 870-e62 (2009).
Gulbransen, B. D., Bains, J. S. & Sharkey, K. A. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J. Neurosci. 30, 6801–6809 (2010).
Gulbransen, B. D. & Sharkey, K. A. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136, 1349–1358 (2009).
Segura, B. J. et al. Lysophosphatidic acid stimulates calcium transients in enteric glia. Neuroscience 123, 687–693 (2004).
Segura, B. J. et al. Sphingosine-1-phosphate mediates calcium signaling in guinea pig enteroglial cells. J. Surg. Res. 116, 42–54 (2004).
Van Nassauw, L. et al. Region-specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton. Neurosci. 126–127, 299–306 (2006).
Christofi, F. L. et al. Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J. Comp. Neurol. 439, 46–64 (2001).
Gulbransen, B. D. et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 18, 600–604 (2012).
Christofi, F. L., Hanani, M., Maudlej, N. & Wood, J. D. Enteric glial cells are major contributors to formation of cyclic AMP in myenteric plexus cultures from adult guinea-pig small intestine. Neurosci. Lett. 159, 107–110 (1993).
Chandrasekharan, B. P. et al. Adenosine 2B receptors (A2BAR) on enteric neurons regulate murine distal colonic motility. FASEB J. 23, 2727–2734 (2009).
Pankratov, Y., Lalo, U., Verkhratsky, A. & North, R. A. Vesicular release of ATP at central synapses. Pflugers Arch. 452, 589–597 (2006).
Kimball, B. C. & Mulholland, M. W. Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism. J. Neurochem. 66, 604–612 (1996).
Sarosi, G. A., Barnhart, D. C., Turner, D. J. & Mulholland, M. W. Capacitative Ca2+ entry in enteric glia induced by thapsigargin and extracellular ATP. Am. J. Physiol. 275, G550–G555 (1998).
Laranjeira, C. et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J. Clin. Invest. 121, 3412–3424 (2011).
MacEachern, S. J., Patel, B. A., McKay, D. M. & Sharkey, K. A. Nitric oxide regulation of colonic epithelial ion transport: a novel role for enteric glia in the myenteric plexus. J. Physiol. 589, 3333–3348 (2011).
Nurgali, K., Furness, J. B. & Stebbing, M. J. Analysis of purinergic and cholinergic fast synaptic transmission to identified myenteric neurons. Neuroscience 116, 335–347 (2003).
White, T. D. Release of ATP from isolated myenteric varicosities by nicotinic agonists. Eur. J. Pharmacol. 79, 333–334 (1982).
Braun, N. et al. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia 45, 124–132 (2004).
Lavoie, E. G. et al. Ectonucleotidases in the digestive system: focus on NTPDase3 localization. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G608–G620 (2011).
Decker, D. A. & Galligan, J. J. Cross-inhibition between nicotinic acetylcholine receptors and P2X receptors in myenteric neurons and HEK-293 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1267–G1276 (2009).
Fletcher, E. L., Clark, M. J. & Furness, J. B. Neuronal and glial localization of GABA transporter immunoreactivity in the myenteric plexus. Cell Tissue Res. 308, 339–346 (2002).
Ruhl, A., Hoppe, S., Frey, I., Daniel, H. & Schemann, M. Functional expression of the peptide transporter PEPT2 in the mammalian enteric nervous system. J. Comp. Neurol. 490, 1–11 (2005).
Nagahama, M., Semba, R., Tsuzuki, M. & Aoki, E. L-arginine immunoreactive enteric glial cells in the enteric nervous system of rat ileum. Biol. Signals Recept. 10, 336–340 (2001).
Zhang, W., Segura, B. J., Lin, T. R., Hu, Y. & Mulholland, M. W. Intercellular calcium waves in cultured enteric glia from neonatal guinea pig. Glia 42, 252–262 (2003).
Johnson, P. J. & Bornstein, J. C. Neurokinin-1 and -3 receptor blockade inhibits slow excitatory synaptic transmission in myenteric neurons and reveals slow inhibitory input. Neuroscience 126, 137–147 (2004).
Grider, J. R. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J. Pharmacol. Exp. Ther. 307, 460–467 (2003).
Copel, C. et al. Activation of neurokinin 3 receptor increases Nav1.9 current in enteric neurons. J. Physiol. 587, 1461–1479 (2009).
Holzer, P. & Holzer-Petsche, U. Tachykinins in the gut. Part II. Roles in neural excitation, secretion and inflammation. Pharmacol. Ther. 73, 219–263 (1997).
Hyland, N. P. & Cryan, J. F. A gut feeling about GABA: focus on GABAB receptors. Front. Pharmacol. 1, 124 (2010).
Kaszaki, J. et al. Kynurenines and intestinal neurotransmission: the role of N-methyl-D-aspartate receptors. J. Neural. Transm. 119, 211–223 (2012).
Gershon, M. D. Manipulating the ENS stem cell in adults. Presented at Digestive Disease Week 2012, San Diego, CA, USA.
Abdo, H. et al. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J. 24, 1082–1094 (2010).
Abdo, H. et al. The omega-6 derivative 15d-PGJ2 is involved in neuroprotection by enteric glial cells against oxidative stress. J. Physiol. 590, 2739–2750 (2012).
Anitha, M. et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J. Clin. Invest. 116, 344–356 (2006).
Rodrigues, D. M., Li, A. Y., Nair, D. G. & Blennerhassett, M. G. Glial cell line-derived neurotrophic factor is a key neurotrophin in the postnatal enteric nervous system. Neurogastroenterol. Motil. 23, e44–e56 (2011).
Kirchgessner, A. L., Liu, M. T. & Alcantara, F. Excitotoxicity in the enteric nervous system. J. Neurosci. 17, 8804–8816 (1997).
Acknowledgements
K. Sharkey is supported by grants from the Canadian Institutes of Health Research (CIHR) and is an Alberta Innovates–Health Solutions (AI–HS) Medical Scientist who holds the Crohn's & Colitis Foundation of Canada (CCFC) Chair in IBD Research at the University of Calgary. B. Gulbransen holds fellowships from the Canadian Association of Gastroenterology (CAG)/CIHR and AI–HS/ CCFC. The authors thank Winnie Ho for technical assistance with Figure 1.
Author information
Authors and Affiliations
Contributions
B. Gulbransen researched data for the article. Both authors contributed equally to discussion of content, writing and review or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gulbransen, B., Sharkey, K. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9, 625–632 (2012). https://doi.org/10.1038/nrgastro.2012.138
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.138
This article is cited by
-
Enteric neurospheres retain the capacity to assemble neural networks with motile and metamorphic gliocytes and ganglia
Stem Cell Research & Therapy (2023)
-
TGFβR-1/ALK5 inhibitor RepSox induces enteric glia-to-neuron transition and influences gastrointestinal mobility in adult mice
Acta Pharmacologica Sinica (2023)
-
CORM-3 alleviates the intestinal injury in a rodent model of hemorrhage shock and resuscitation: roles of GFAP-positive glia
Journal of Molecular Histology (2023)
-
Microbiota-dependent presence of murine enteric glial cells requires myeloid differentiation primary response protein 88 signaling
Journal of Biosciences (2023)
-
Effects of experimental ulcerative colitis on myenteric neurons in P2X7-knockout mice
Histochemistry and Cell Biology (2023)