Key Points
-
Translational control is a widespread means of regulating gene expression in development and cellular processes. It enables highly dynamic responses to external signals and contributes to protein targeting.
-
Translational control mechanisms target many steps of protein synthesis and influence many components of the core translational machinery.
-
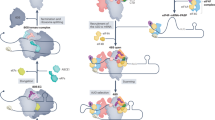
Eukaryotic translation initiation factor 4E (eIF4E), an initiation factor that recognizes the 5′ cap structure of mRNA and assembles a complex that is necessary for ribosome recruitment, is targeted by translational repressors called eIF4E-binding proteins (4EBPs). 4EBPs are implicated in numerous cellular, developmental and physiological processes.
-
Many other translational regulators operate through effects on the poly(A) tail length of targeted mRNAs, and others affect later steps of translation, such as subunit joining and elongation. Some translational repressors operate by sequestering mRNAs into inactive RNA granules.
-
Localization of mRNA often complements translational control by concentrating the mRNA in a cytoplasmic space where it is translationally active.
-
Defects in translational control have been linked to many human diseases, indicating its central role in developmental and cellular processes.
Abstract
Growing evidence indicates that translational control of specific mRNAs contributes importantly to genetic regulation across the breadth of cellular and developmental processes. Synthesis of protein from a specific mRNA can be controlled by RNA-binding proteins at the level of translational initiation and elongation, and translational control is also sometimes coupled to mRNA localization mechanisms. Recent discoveries from invertebrate and vertebrate systems have uncovered novel modes of translational regulation, have provided new insights into how specific regulators target the general translational machinery and have identified several new links between translational control and human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Loya, C. M., Van Vactor, D. & Fulga, T. A. Understanding neuronal connectivity through the post-transcriptional toolkit. Genes Dev. 24, 625–635 (2010).
Schwanhausser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011). This is a comprehensive and careful study that documents the widespread nature of post-transcriptional genetic regulation.
Lecuyer, E. et al. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell 131, 174–187 (2007).
Esteller, M. Non-coding RNAs in human disease. Nature Rev. Genet. 12, 861–874 (2011).
Krol, J., Loedige, I. & Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nature Rev. Genet. 11, 597–610 (2010).
Filbin, M. E. & Kieft, J. S. Toward a structural understanding of IRES RNA function. Curr. Opin. Struct. Biol. 19, 267–276 (2009).
Shatsky, I. N., Dmitriev, S. E., Terenin, I. M. & Andreev, D. E. Cap- and IRES-independent scanning mechanism of translation initiation as an alternative to the concept of cellular IRESs. Mol. Cells 30, 285–293 (2010).
Jackson, R. J., Hellen, C. U. & Pestova, T. V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nature Rev. Mol. Cell Biol. 11, 113–127 (2010).
Sonenberg, N. & Hinnebusch, A. G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136, 731–745 (2009).
Cridge, A. G. et al. Identifying eIF4E-binding protein translationally-controlled transcripts reveals links to mRNAs bound by specific PUF proteins. Nucleic Acids Res. 38, 8039–8050 (2010).
Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 (2004).
Rapley, J., Oshiro, N., Ortiz-Vega, S. & Avruch, J. The mechanism of insulin-stimulated 4E-BP protein binding to mammalian target of rapamycin (mTOR) complex 1 and its contribution to mTOR complex 1 signaling. J. Biol. Chem. 286, 38043–38053 (2011).
Dowling, R. J. et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328, 1172–1176 (2010).
Le Bacquer, O. et al. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J. Clin. Invest. 117, 387–396 (2007).
Olson, K. E., Booth, G. C., Poulin, F., Sonenberg, N. & Beretta, L. Impaired myelopoiesis in mice lacking the repressors of translation initiation, 4E-BP1 and 4E-BP2. Immunology 128, e376–e384 (2009).
Demontis, F. & Perrimon, N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 143, 813–825 (2010). This is a comprehensive study of muscle ageing that implicates 4EBPs in the removal of damaged and aggregated protein.
Zid, B. M. et al. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 139, 149–160 (2009).
Cao, Q. & Richter, J. D. Dissolution of the maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J. 21, 3852–3862 (2002).
Groisman, I., Jung, M. Y., Sarkissian, M., Cao, Q. & Richter, J. D. Translational control of the embryonic cell cycle. Cell 109, 473–483 (2002).
Barnard, D. C., Cao, Q. & Richter, J. D. Differential phosphorylation controls Maskin association with eukaryotic translation initiation factor 4E and localization on the mitotic apparatus. Mol. Cell Biol. 25, 7605–7615 (2005).
Cao, Q., Kim, J. H. & Richter, J. D. CDK1 and calcineurin regulate Maskin association with eIF4E and translational control of cell cycle progression. Nature Struct. Mol. Biol. 13, 1128–1134 (2006).
Nakamura, A., Sato, K. & Hanyu-Nakamura, K. Drosophila Cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev. Cell 6, 69–78 (2004).
Nelson, M. R., Leidal, A. M. & Smibert, C. A. Drosophila Cup is an eIF4E-binding protein that functions in Smaug-mediated translational repression. EMBO J. 23, 150–159 (2004).
Igreja, C. & Izaurralde, E. CUP promotes deadenylation and inhibits decapping of mRNA targets. Genes Dev. 25, 1955–1967 (2011).
Jeske, M., Moritz, B., Anders, A. & Wahle, E. Smaug assembles an ATP-dependent stable complex repressing nanos mRNA translation at multiple levels. EMBO J. 30, 90–103 (2011).
Richter, J. D. & Klann, E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 23, 1–11 (2009).
Napoli, I. et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell 134, 1042–1054 (2008).
Rosettani, P., Knapp, S., Vismara, M. G., Rusconi, L. & Cameron, A. D. Structures of the human eIF4E homologous protein, h4EHP, in its m7GTP-bound and unliganded forms. J. Mol. Biol. 368, 691–705 (2007).
Cho, P. F. et al. A new paradigm for translational control: inhibition via 5′–3′ mRNA tethering by Bicoid and the eIF4E cognate 4EHP. Cell 121, 411–423 (2005).
Cho, P. F. et al. Cap-dependent translational inhibition establishes two opposing morphogen gradients in Drosophila embryos. Curr. Biol. 16, 2035–2041 (2006).
Villaescusa, J. C. et al. Cytoplasmic Prep1 interacts with 4EHP inhibiting Hoxb4 translation. PLoS ONE 4, e5213 (2009).
Antonchuk, J., Sauvageau, G. & Humphries, R. K. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 109, 39–45 (2002).
Okumura, F., Zou, W. & Zhang, D. E. ISG15 modification of the eIF4E cognate 4EHP enhances cap structure-binding activity of 4EHP. Genes Dev. 21, 255–260 (2007).
Hernandez, G. et al. Functional analysis of seven genes encoding eight translation initiation factor 4E (eIF4E) isoforms in Drosophila. Mech. Dev. 122, 529–543 (2005).
Tadros, W. et al. SMAUG is a major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase. Dev. Cell 12, 143–155 (2007).
Chicoine, J. et al. Bicaudal-C recruits CCR4-NOT deadenylase to target mRNAs and regulates oogenesis, cytoskeletal organization, and its own expression. Dev. Cell 13, 691–704 (2007).
Kadyrova, L. Y., Habara, Y., Lee, T. H. & Wharton, R. P. Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development 134, 1519–1527 (2007).
Belloc, E. & Mendez, R. A deadenylation negative feedback mechanism governs meiotic metaphase arrest. Nature 452, 1017–1021 (2008).
Rouget, C. et al. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature 467, 1128–1132 (2010).
de Moor, C. H. & Richter, J. D. Cytoplasmic polyadenylation elements mediate masking and unmasking of cyclin B1 mRNA. EMBO J. 18, 2294–2303 (1999).
Charlesworth, A., Cox, L. L. & MacNicol, A. M. Cytoplasmic polyadenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes. J. Biol. Chem. 279, 17650–17659 (2004).
Keady, B. T., Kuo, P., Martinez, S. E., Yuan, L. & Hake, L. E. MAPK interacts with XGef and is required for CPEB activation during meiosis in Xenopus oocytes. J. Cell Sci. 120, 1093–1103 (2007).
Mendez, R. et al. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404, 302–307 (2000).
Tay, J. & Richter, J. D. Germ cell differentiation and synaptonemal complex formation are disrupted in CPEB knockout mice. Dev. Cell 1, 201–213 (2001).
Vilborg, A., Wilhelm, M. T. & Wiman, K. G. Regulation of tumor suppressor p53 at the RNA level. J. Mol. Med. 88, 645–652 (2010).
Burns, D. M. & Richter, J. D. CPEB regulation of human cellular senescence, energy metabolism, and p53 mRNA translation. Genes Dev. 22, 3449–3460 (2008).
Katoh, T. et al. Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes Dev. 23, 433–438 (2009).
Burns, D. M., D.'Ambrogio, A., Nottrott, S. & Richter, J. D. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature 473, 105–108 (2011). In this study, the authors demonstrate that a miRNA can be a target for cytoplasmic polyadenylation and explain why CPEB, which promotes polyadenylation, nevertheless negatively regulates TP53 mRNA.
Wethmar, K., Smink, J. J. & Leutz, A. Upstream open reading frames: molecular switches in (patho)physiology. Bioessays 32, 885–893 (2010).
Beckmann, K., Grskovic, M., Gebauer, F. & Hentze, M. W. A dual inhibitory mechanism restricts msl-2 mRNA translation for dosage compensation in Drosophila. Cell 122, 529–540 (2005).
Duncan, K. E., Strein, C. & Hentze, M. W. The SXL-UNR corepressor complex uses a PABP-mediated mechanism to inhibit ribosome recruitment to msl-2 mRNA. Mol. Cell 36, 571–582 (2009).
Medenbach, J., Seiler, M. & Hentze, M. W. Translational control via protein-regulated upstream open reading frames. Cell 145, 902–913 (2011).
Naarmann, I. S. et al. DDX6 recruits translational silenced human reticulocyte 15-lipoxygenase mRNA to RNP granules. RNA 16, 2189–2204 (2010).
Johnstone, O. & Lasko, P. Interaction with eIF5B is essential for Vasa function during development. Development 131, 4167–4178 (2004).
Liu, N., Han, H. & Lasko, P. Vasa promotes Drosophila germline stem cell differentiation by activating mei-P26 translation by directly interacting with a (U)-rich motif in its 3′ UTR. Genes Dev. 23, 2742–2752 (2009).
Stefani, G., Fraser, C. E., Darnell, J. C. & Darnell, R. B. Fragile X mental retardation protein is associated with translating polyribosomes in neuronal cells. J. Neurosci. 24, 7272–7276 (2004).
Darnell, J. C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011). This study identifies 842 potential mRNA targets for FMRP and provides evidence that FMRP stalls ribosomes and inhibits elongation.
Balagopal, V. & Parker, R. Stm1 modulates translation after 80S formation in Saccharomyces cerevisiae. RNA 17, 835–842 (2011).
Ryazanov, A. G. & Davydova, E. K. Mechanism of elongation factor 2 (EF-2) inactivation upon phosphorylation. Phosphorylated EF-2 is unable to catalyze translocation. FEBS Lett. 251, 187–190 (1989).
Park, S. et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59, 70–83 (2008).
Weatherill, D. B. et al. Compartment-specific, differential regulation of eukaryotic elongation factor 2 and its kinase within Aplysia sensory neurons. J. Neurochem. 117, 841–855 (2011).
Majmundar, A. J., Wong, W. J. & Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 (2010).
Chen, P. J. & Huang, Y. S. CPEB2-eEF2 interaction impedes HIF-1α RNA translation. EMBO J. 31, 959–971 (2011).
Hussey, G. S. et al. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol. Cell 41, 419–431 (2011).
Friend, K. et al. A conserved PUF-Ago-eEF1A complex attenuates translation elongation. Nature Struct. Mol. Biol. 19, 176–183 (2012).
Takagi, M., Absalon, M. J., McLure, K. G. & Kastan, M. B. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell 123, 49–63 (2005).
Chen, J. & Kastan, M. B. 5′–3′-UTR interactions regulate p53 mRNA translation and provide a target for modulating p53 induction after DNA damage. Genes Dev. 24, 2146–2156 (2010).
Lee, J. T. & Gu, W. The multiple levels of regulation by p53 ubiquitination. Cell Death Differ. 17, 86–92 (2010).
Ofir-Rosenfeld, Y., Boggs, K., Michael, D., Kastan, M. B. & Oren, M. Mdm2 regulates p53 mRNA translation through inhibitory interactions with ribosomal protein L26. Mol. Cell 32, 180–189 (2008).
McGowan, K. A. et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nature Genet. 40, 963–970 (2008).
Barkic, M. et al. The p53 tumor suppressor causes congenital malformations in RPL24-deficient mice and promotes their survival. Mol. Cell Biol. 29, 2489–2504 (2009).
Cmejla, R., Cmejlova, J., Handrkova, H., Petrak, J. & Pospisilova, D. Ribosomal protein S17 gene (RPS17) is mutated in Diamond–Blackfan anemia. Hum. Mutat. 28, 1178–1182 (2007).
Draptchinskaia, N. et al. The gene encoding ribosomal protein S19 is mutated in Diamond–Blackfan anaemia. Nature Genet. 21, 169–175 (1999).
Farrar, J. E. et al. Abnormalities of the large ribosomal subunit protein, RPL35a, in Diamond–Blackfan anemia. Blood 112, 1582–1592 (2008).
Gazda, H. T. et al. Ribosomal protein S24 gene is mutated in Diamond–Blackfan anemia. Am. J. Hum. Genet. 79, 1110–1118 (2006).
Gazda, H. T. et al. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond–Blackfan anemia patients. Am. J. Hum. Genet. 83, 769–780 (2008).
Horos, R. et al. Ribosomal deficiencies in Diamond–Blackfan anemia impair translation of transcripts essential for differentiation of murine and human erythroblasts. Blood 119, 262–272 (2012).
Amsterdam, A. et al. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol. 2, e139 (2004).
Duan, J. et al. Knockdown of ribosomal protein S7 causes developmental abnormalities via p53 dependent and independent pathways in zebrafish. Int. J. Biochem. Cell Biol. 43, 1218–1227 (2011).
Kondrashov, N. et al. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell 145, 383–397 (2011).
Jack, K. et al. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol. Cell 44, 660–666 (2011).
Bellodi, C., Kopmar, N. & Ruggero, D. Deregulation of oncogene-induced senescence and p53 translational control in X-linked dyskeratosis congenita. EMBO J. 29, 1865–1876 (2010).
Bellodi, C. et al. Loss of function of the tumor suppressor DKC1 perturbs p27 translation control and contributes to pituitary tumorigenesis. Cancer Res. 70, 6026–6035 (2010).
Higa-Nakamine, S. et al. Loss of ribosomal RNA modification causes developmental defects in zebrafish. Nucleic Acids Res. 40, 391–398 (2012).
Anderson, P. & Kedersha, N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nature Rev. Mol. Cell Biol. 10, 430–436 (2009).
Thomas, M. G., Loschi, M., Desbats, M. A. & Boccaccio, G. L. RNA granules: the good, the bad and the ugly. Cell Signal 23, 324–334 (2011).
Besse, F., Lopez de Quinto, S., Marchand, V., Trucco, A. & Ephrussi, A. Drosophila PTB promotes formation of high-order RNP particles and represses oskar translation. Genes Dev. 23, 195–207 (2009).
Sawicka, K., Bushell, M., Spriggs, K. A. & Willis, A. E. Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochem. Soc. Trans. 36, 641–647 (2008).
Snee, M. J. & Macdonald, P. M. Dynamic organization and plasticity of sponge bodies. Dev. Dyn. 238, 918–930 (2009).
Knoch, K. P. et al. Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis. Nature Cell Biol. 6, 207–214 (2004).
Martin, K. C. & Ephrussi, A. mRNA localization: gene expression in the spatial dimension. Cell 136, 719–730 (2009).
Kugler, J. M. & Lasko, P. Localization, anchoring and translational control of oskar, gurken, bicoid and nanos mRNA during Drosophila oogenesis. Fly 3, 15–28 (2009).
Mili, S., Moissoglu, K. & Macara, I. G. Genome-wide screen reveals APC-associated RNAs enriched in cell protrusions. Nature 453, 115–119 (2008).
Sutton, M. A. & Schuman, E. M. Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127, 49–58 (2006).
Doyle, M. & Kiebler, M. A. Mechanisms of dendritic mRNA transport and its role in synaptic tagging. EMBO J. 30, 3540–3552 (2011).
Navarro, C., Puthalakath, H., Adams, J. M., Strasser, A. & Lehmann, R. Egalitarian binds dynein light chain to establish oocyte polarity and maintain oocyte fate. Nature Cell Biol. 6, 427–435 (2004).
Dienstbier, M., Boehl, F., Li, X. & Bullock, S. L. Egalitarian is a selective RNA-binding protein linking mRNA localization signals to the dynein motor. Genes Dev. 23, 1546–1558 (2009) This study provides a detailed and complete mechanism for dynein-mediated mRNA localization in D. melanogaster blastoderm cells.
Hoogenraad, C. C. et al. Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J. 20, 4041–4054 (2001).
Hoogenraad, C. C. et al. Bicaudal D induces selective dynein-mediated microtubule minus end-directed transport. EMBO J. 22, 6004–6015 (2003).
Hachet, O. & Ephrussi, A. Splicing of oskar RNA in the nucleus is coupled to its cytoplasmic localization. Nature 428, 959–963 (2004).
Jambor, H., Brunel, C. & Ephrussi, A. Dimerization of oskar 3′ UTRs promotes hitchhiking for RNA localization in the Drosophila oocyte. RNA 17, 2049–2057 (2011).
Sinsimer, K. S., Jain, R. A., Chatterjee, S. & Gavis, E. R. A late phase of germ plasm accumulation during Drosophila oogenesis requires lost and rumpelstiltskin. Development 138, 3431–3440 (2011).
Tanaka, T., Kato, Y., Matsuda, K., Hanyu-Nakamura, K. & Nakamura, A. Drosophila Mon2 couples Oskar-induced endocytosis with actin remodeling for cortical anchorage of the germ plasm. Development 138, 2523–2532 (2011).
Vanzo, N., Oprins, A., Xanthakis, D., Ephrussi, A. & Rabouille, C. Stimulation of endocytosis and actin dynamics by Oskar polarizes the Drosophila oocyte. Dev. Cell 12, 543–555 (2007).
Zimyanin, V. L. et al. In vivo imaging of oskar mRNA transport reveals the mechanism of posterior localization. Cell 134, 843–853 (2008).
Chao, J. A. et al. ZBP1 recognition of β-actin zipcode induces RNA looping. Genes Dev. 24, 148–158 (2010).
Shen, Z., St-Denis, A. & Chartrand, P. Cotranscriptional recruitment of She2p by RNA pol II elongation factor Spt4-Spt5/DSIF promotes mRNA localization to the yeast bud. Genes Dev. 24, 1914–1926 (2010).
Chung, S. & Takizawa, P. A. Multiple Myo4 motors enhance ASH1 mRNA transport in Saccharomyces cerevisiae. J. Cell Biol. 189, 755–767 (2010).
Muller, M. et al. A cytoplasmic complex mediates specific mRNA recognition and localization in yeast. PLoS Biol. 9, e1000611 (2011).
Ingolia, N. T., Ghaemmaghami, S., Newman, J. R. & Weissman, J. S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324, 218–223 (2009). This article reports a technology that promises to enable precise and comprehensive studies of translation in living cells.
Oeffinger, M. et al. Comprehensive analysis of diverse ribonucleoprotein complexes. Nature Meth. 4, 951–956 (2007).
Tadros, W. & Lipshitz, H. D. The maternal-to-zygotic transition: a play in two acts. Development 136, 3033–3042 (2009).
Lasko, P. Posttranscriptional regulation in Drosophila oocytes and early embryos. Wiley Interdiscip. Rev. RNA 2, 408–416 (2011).
Piccioni, F., Zappavigna, V. & Verrotti, A. C. A cup full of functions. RNA Biol. 2, 125–128 (2005).
Chekulaeva, M., Hentze, M. W. & Ephrussi, A. Bruno acts as a dual repressor of oskar translation, promoting mRNA oligomerization and formation of silencing particles. Cell 124, 521–533 (2006).
Zappavigna, V., Piccioni, F., Villaescusa, J. C. & Verrotti, A. C. Cup is a nucleocytoplasmic shuttling protein that interacts with the eukaryotic translation initiation factor 4E to modulate Drosophila ovary development. Proc. Natl Acad. Sci. USA 101, 14800–14805 (2004).
Cencic, R. et al. Reversing chemoresistance by small molecule inhibition of the translation initiation complex eIF4F. Proc. Natl Acad. Sci. USA 108, 1046–1051 (2011).
Imai, Y. et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 27, 2432–2443 (2008).
Yang, Y. et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc. Natl Acad. Sci. USA 103, 10793–10798 (2006).
Tain, L. S. et al. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nature Neurosci. 12, 1129–1135 (2009).
De Rubeis, S. & Bagni, C. Regulation of molecular pathways in the fragile X syndrome: insights into autism spectrum disorders. J. Neurodev. Disord. 3, 257–269 (2011).
Sahoo, T. et al. Prader–Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nature Genet. 40, 719–721 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- 5′ cap structure
-
A 7-methylguanosine residue that is enzymatically added to the 5′ end of an mRNA and linked through a 5′–5′ triphosphate bridge.
- Mammalian target of rapamycin complex 1
-
(mTORC1). A signalling complex that senses nutrient abundance and regulates cell growth and proliferation accordingly.
- Pachytene
-
The stage during the first meiotic division when chromosomal crossing-over and recombination occurs.
- Synaptonemal complex
-
A protein structure that forms between homologous chromosomes during the first meiotic prophase and that facilitates chromosome pairing.
- Ribonucleoproteins
-
Multimolecular complexes that contain both RNAs and proteins.
- Heterogeneous nuclear ribonucleoprotein E1
-
(hnRNPE1). An RNA-binding protein with functions in precursor mRNA processing and in regulating mRNA stability and translation. Despite its name, it is present and functional both in nuclei and in the cytoplasm.
- Polyribosomes
-
Actively translated mRNAs that are associated with multiple ribosomes, each elongating a different nascent polypeptide chain.
- High-throughput sequencing coupled with crosslinking and immunoprecipitation
-
(HITS–CLIP). A technique in which mRNAs associated with a particular protein or in a ribonucleoprotein complex are recovered by co-immunoprecipitation and analysed by deep sequencing.
- Normoxia
-
A physiological condition in which oxygen levels are sufficient and not limiting for metabolic processes.
- MDM2
-
An E3 ubiquitin ligase that recognizes and destabilizes p53.
- Prader–Willi syndrome
-
A rare genetic disorder that results in obesity and reductions in muscle tone, cognitive capacity and production of sex hormones.
- Homeotic transformation
-
In developmental biology, a situation that is often caused by a mutation or an alteration in gene expression whereby precursors to a particular cell, tissue or organ type develop instead into a different one.
- Homeobox gene
-
(HOX gene). One of a set of genes that encodes a particular type of transcription factor and that is implicated in establishing many developmental fates, including the identity of body segments along the anterior–posterior axis.
- Processing bodies
-
Also called P bodies, these constitute a type of RNA granule that is linked to cytoplasmic RNA decay pathways.
- Ribosome profiling
-
A technique for measuring translation of many species of mRNA simultaneously in vivo.
Rights and permissions
About this article
Cite this article
Kong, J., Lasko, P. Translational control in cellular and developmental processes. Nat Rev Genet 13, 383–394 (2012). https://doi.org/10.1038/nrg3184
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3184
This article is cited by
-
Central dogma rates and the trade-off between precision and economy in gene expression
Nature Communications (2019)
-
A ribosomal protein S5 isoform is essential for oogenesis and interacts with distinct RNAs in Drosophila melanogaster
Scientific Reports (2019)
-
Heterogeneity and specialized functions of translation machinery: from genes to organisms
Nature Reviews Genetics (2018)
-
Comparative ribosome profiling uncovers a dominant role for translational control in Toxoplasma gondii
BMC Genomics (2017)
-
Rps26 directs mRNA-specific translation by recognition of Kozak sequence elements
Nature Structural & Molecular Biology (2017)