Key Points
-
Mutations in the X-linked coagulation factor VIII (F8) gene lead to haemophilia A of different grades of severity in humans.
-
Approximately half the severe cases are due to intron-22 inversions. For smaller deletions, insertions or point mutations, the degree of severity depends on the function of the affected domains. Surprisingly, in about 4% of the haemophiliacs no mutation can be found in the coding exons and their flanking sequences.
-
Several hundred missense mutations in the F8 gene have been identified; these are used to understand the function of the various domains of the F8 protein.
-
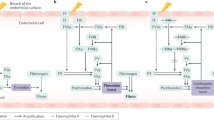
Many proteins interact with F8 during its transport through cellular compartments, and regulate its activation and inactivation. Mutations in genes that code for these proteins lead to haemophilia-like symptoms (in particular mutations in the VWD, LMAN1 and MCFD2 genes).
-
Because ∼2% of haemophiliacs do not carry mutations in the coding regions of the F8 gene, further efforts are needed to analyse the F8 mRNA (and the corresponding cDNA) from its ectopic expression in lymphocytes.
-
Haemophilia A has been a leading model in the field of gene therapy; however, the disappointingly low levels and short time range of F8 expression need to be increased.
-
Current research focuses on increasing the yield of recombinant F8, improving its transport, extending its half-life and reducing its antigenicity.
Abstract
Haemophilia is caused by hundreds of different mutations and manifests itself in clinical conditions of varying severity. Despite being inherited in monogenic form, the clinical features of haemophilia can be influenced by other genetic factors, thereby confounding the boundary between monogenic and multifactorial disease. Unlike sufferers of other genetic diseases, haemophiliacs can be treated successfully by intravenous substitution of coagulation factors. Haemophilia is also the most attractive model for developing gene-therapy protocols, as the normal life expectancy of haemophiliacs allows the side effects of gene therapy, as well as its efficiency, to be monitored over long periods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Otto, J. C. An account of an haemorrhagic disposition existing in certain families. Med. Repos. 6, 1–4 (1803).
Hoppf, F. Über die Hämophilie oder die erbliche Anlage zu tödlichen Blutungen. Thesis, Univ. Zurich (1828).
Aggeler, P. M. et al. Plasma thromboplastin component (PTC) deficiency: a new disease resembling hemophilia. Proc. Soc. Exp. Biol. Med. 79, 692–694 (1952).
Biggs, R. et al. Christmas disease: a condition previously mistaken for haemophilia. Brit. Med. J. 2, 1378–1382 (1952).
Ingram, G. I. C. The history of haemophilia. J. Clin. Path. 29, 469–479 (1976).
Rotblat, F. et al. Monoclonal antibodies to human procoagulant factor VIII. J. Lab. Clin. Med. 101, 736–746 (1983).
Hilgartner, M. W. The need for recombinant factor FVIII. Historical background and rationale. Semin. Hematol. 28, 6–9 (1991).
Gitschier, J. et al. Characterization of the human factor VIII gene. Nature 312, 326–330 (1984). Characterization of the large F8 gene in humans — 20 years before the human genome sequence was published.
Oldenburg, J., Ananyeva, N. M. & Saenko, E. L. Molecular basis of haemophilia A. Haemophilia 10 (Suppl. 4), 133–139 (2004).
McGlynn, L. K., Mueller, C. R., Begbie, M., Notley, C. R. & Lillicrap, D. Role of the liver-enriched transcription factor hepatocyte nuclear factor-1 in transcriptional regulation of the factor VIII gene. Mol. Cell. Biol. 16, 1936–1945 (1996).
Ghosh, K. Management of haemophilia and its complications in developing countries. Clin. Lab. Haem. 26, 243–251 (2004).
Antonarakis, S. E., Kazazian, H. H. & Tuddenham, G. D. Molecular etiology of Factor VIII deficiency in hemophilia. Hum. Mutat. 5, 1–22 (1995).
Nichols, W. C. et al. Mutations in the ER–Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell 93, 61–70 (1998).
Nichols, W. C. et al. ERGIC-53 gene structure and mutation analysis in 19 combined factors V and VIII deficiency families. Blood 93, 2261–2266 (1999).
Zhang, B. et al. Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex. Nature Genet. 34, 220–225 (2003).
Zhang, B. & Ginsburg, D. Familial multiple coagulation factor deficiencies: new biologic insight from rare genetic bleeding disorders. J. Thromb. Haemost. 2, 1564–1572 (2004).
Nishino, M., Girma, J. P., Rothschild, C., Fressinaud, E. & Meyer, D. New variant of von Willebrand disease with defective binding to factor VIII. Blood 74, 1591–1599 (1989).
Gaucher, C., Mercier, B., Jorieux, S., Oufkir, D. & Mazurier, C. Identification of two point mutations in the von Willebrand factor gene of three families with the 'Normandy' variant of von Willebrand disease. Br. J. Haematol. 78, 506–514 (1991).
Hilbert, L., D'Oiron, R., Fressinaud, E., Meyer, D. & Mazurier, C. INSERM network on molecular abnormalities in von Willebrand disease. First identification and expression of a type 2N von Willebrand disease mutation (E1078K) located in exon 25 of von Willebrand factor gene. J. Thromb. Haemost. 2, 2271–2273 (2004).
Schneppenheim, R. et al. Results of a screening for von Willebrand disease type 2N in patients with suspected haemophilia A or von Willebrand disease type 1. Thromb. Haemostas. 76, 598–602 (1996).
Klopp, N., Oldenburg, J., Uen, C., Schneppenheim, R. & Graw, J. 11 hemophilia A patients without mutations in the factor VIII encoding gene. Thromb. Haemost. 88, 357–360 (2002).
Peerlinck, K. et al. A patient with von Willebrand's disease characterized by a compound heterozygosity for a substitution of Arg854 by Gln in the putative factor-VIII-binding domain of von Willebrand factor (vWF) on one allele and very low levels of mRNA from the second vWF allele. Br. J. Haematol. 80, 358–363 (1992).
Harvey, D. & Lowe, G. M. Factor V Leiden: association with venous thromboembolism in pregnancy and screening issues. Br. J. Biomed. Sci. 61, 157–164 (2004).
Zivelin, A. et al. A single genetic origin for the common prothrombotic G20210A polymorphism in the prothrombin gene. Blood 92, 1119–1124 (1998).
Ahmed, R., Kannan, M., Choudhry, V. P. & Saxena, R. Does the MTHFR 677T allele alter the clinical phenotype in severe haemophilia A? Thromb. Res. 109, 71–72 (2003).
van Dijk, K., van der Bom, J. G., Fischer, K., Grobbee, D. E. & van den Berg, H. M. Do prothrombotic factors influence clinical phenotype of severe haemophilia? A review of the literature. Thromb. Haemost. 92, 305–310 (2004).
Poustka, A. et al. Physical map of human Xq27-qter: localizing the region of the fragile X mutation. Proc. Natl Acad. Sci. USA 88, 8302–8306 (1991).
Freije, D. & Schlessinger, D. A 1.6-Mb contig of yeast artificial chromosomes around the human factor VIII gene reveals three regions homologous to probes for the DXS115 locus and two for the DXYS64 locus. Am. J. Hum. Genet. 51, 66–80 (1992).
Levinson, B., Kenwrick, S., Lakich, D., Hammonds, G. Jr, Gitschier, J. A transcribed gene in an intron of the human factor VIII gene. Genomics 7, 1–11 (1990). This paper describes the surprising discovery of a second transcribed gene in the large intron of F8 , one of only a few findings of its kind in eukaryotes.
Levinson, B. et al. Sequence of the human factor VIII-associated gene is conserved in the mouse. Genomics 13, 862–865 (1992).
Peters, M. F. & Ross, C. A. Isolation of a 40-kDa Huntingtin-associated protein. J. Biol. Chem. 276, 3188–3194 (2001).
Valleix, S. et al. Expression of human F8B, a gene nested within the coagulation factor VIII gene, produces multiple eye defects and developmental alterations in chimeric and transgenic mice. Hum. Mol. Genet. 8, 1291–1301 (1999).
Brinke, A. et al. Two chimaeric transcription units result from an inversion breaking intron 1 of the factor VIII gene and a region reportedly affected by reciprocal translocations in T-cell leukaemia. Hum. Mol. Genet. 5, 1945–1951 (1996).
Bagnall, R. D., Waseem, N., Green, P. M. & Gianelli, F. Recurrent inversion breaking intron 1 of the factor VIII gene is a frequent cause of severe hemophilia A. Blood 99, 168–174 (2002). This paper demonstrates the presence of intron-1 inversions in the human F8 gene; the inversions were explained as being caused by homologous recombination between intron 1 and a homologous sequence outside the F8 gene.
Salviato, R., Belvini, D., Radossi, P. & Tagariello, G. Factor VIII gene intron-1 inversion: lower than expected prevalence in Italian haemophiliac severe patients. Haemophilia 10, 194–196 (2004).
Naylor, J. A. et al. Investigation of the factor VIII intron 22 repeated region (int22h) and the associated inversion junctions. Hum. Mol. Genet. 4, 1217–1224 (1995).
Rossiter, J. P. et al. Factor VIII gene inversions causing severe hemophilia A originate almost exclusively in male germ cells. Hum. Mol. Genet. 3, 1035–1039 (1994).
Vidal, F., Farssac, E., Tusell, J., Puig, L. & Gallardo, D. First molecular characterization of an unequal homologous Alu-mediated recombination event responsible for hemophilia. Thromb. Haemost. 88, 12–16 (2002).
Rossetti, L. C., Goodeve, A., Larripa, I. B. & De Brasi, C. D. Homeologous recombination between AluSx-sequences as a cause of Hemophilia. Hum. Mut. 24, 440 (2004).
Nakaya, S. M., Hsu, T. C., Geraghty, S. J., Manco-Johnson, M. J. & Thompson, A. R. Severe hemophilia A due to a 1.3 kb factor VIII gene deletion including exon 24: homologous recombination between 41 bp within an Alu repeat sequence in introns 23 and 24. J. Thromb. Haemost. 2, 1941–1945 (2004).
Mukherjee, S., Mukhopadhyay, A., Chaudhuri, K. & Ray, K. Analysis of haemophilia B database and strategies for common point mutations in the factor IX gene. Haemophilia 9, 187–192 (2003).
Young, M. et al. Partial correction of a severe molecular defect in hemophilia A, because of errors during expression of the factor VIII gene. Am. J. Hum. Genet. 60, 565–573 (1997).
Burns, D. P. W. & Temin, H. M. High rates of frameshift mutations within homo-oligomeric runs during a single cycle of retroviral replication. J. Virol. 68, 4196–4203 (1994).
El-Maarri, O. et al. Analysis of mRNA in hemophilia A patients with undetectable mutations reveals normal splicing in the factor VIII gene. J. Thromb. Haemost. 3, 332–339 (2005).
Figueiredo, M. S. & Brownlee, G. G. cis-Acting elements and transcription factors involved in the promoter activity of the human factor VIII gene. J. Biol. Chem. 270, 11828–11838 (1995).
Begbie, M., Notley, C., Tinlin, S., Sawyer, L. & Lillicrap, D. The factor VIII acute phase response requires the participation of NFκB and C/EBP. Thromb. Haemost. 84, 216–222 (2000).
Wood, W. I. et al. Expression of active human factor VIII from recombinant DNA clones. Nature 312, 330–337 (1984). The authors describe for the first time the successful expression of recombinant F8, providing the means to replace the use of plasma-derived factors in the treatment of haemophilia.
Roelse, J. C. et al. Altered sensitivity towards factor IXa explains assay discrepancies in plasma of haemophilia A patients with a Glu720Lys substitution in factor VIII. Thromb. Haemost. 82, 5a (1999).
Goodeve, A. C. et al. Unusual discrepant factor VIII:C assays in haemophilia A patients with TYR346CYS and GLU321LYS FVIII gene mutations. Thromb. Haemost. (Suppl.), P1370 (2001).
Mumford, A. D. et al. A Tyr346–Cys substitution in the interdomain acidic region a1 of factor VIII in an individual with factor VIII:C assay discrepancy. Br. J. Haemotol. 118, 589–594 (2002).
Pattinson, J. K., McVey, J. H., Boon, M., Ajani, A. & Tuddenham, E. G. CRM+ haemophilia A due to a missense mutation (372–Cys) at the internal heavy chain thrombin cleavage site. Br. J. Haematol. 75, 73–77 (1990).
Arai, M. et al. Direct characterization of factor VIII in plasma: detection of a mutation altering a thrombin cleavage site (arginine–372histidine). Proc. Natl Acad. Sci. USA 86, 4277–4281 (1989).
Arai, M. et al. Characterization of a thrombin cleavage site mutation (Arg1689 to Cys) in the factor VIII gene of two unrelated patients with cross-reacting material-positive hemophilia A. Blood 75, 384–389 (1990).
Higuchi, M. et al. Characterization of mutations in the factor VIII gene by direct sequencing of amplified genomic DNA. Genomics 61, 65–71 (1990).
Gitschier, J., Kogan, S., Levinson, B. & Tuddenham, E. G. Mutations of factor VIII cleavage sites in hemophilia A. Blood 72, 1022–1028 (1988).
Hakeos, W. H. et al. Hemophilia A mutations within the factor VIII A2–A3 subunit interface destabilize factor VIIIa and cause one-stage/two-stage activity discrepancy. Thromb. Haemost. 88, 781–787 (2002).
Keeling, D. M. et al. Diagnostic importance of the two-stage factor VIII:C assay demonstrated by a case of mild haemophilia associated with His1954–Leu substitution in the factor VIII A3 domain. Br. J. Haematol. 105, 1123–1126 (1999).
Rudzki, Z. et al. Mutations in a subgroup of patients with mild haemophilia A and a familial discrepancy between the one-stage and two-stage factor VIII:C methods. Br. J. Haematol. 94, 400–406 (1996).
Oldenburg, J. & Schwaab, R. Molecular biology of blood coagulation. Semin. Thromb. Hemost. 27, 313–324 (2001).
Ananyeva, N. M. et al. Inhibitors in hemophilia A: mechanisms of inhibition, management and perspectives. Blood Coag. Fibrinol. 15, 109–124 (2004).
Amano, K. et al. The molecular basis for cross-reacting material-positive hemophilia A due to missense mutations within the A2-domain of factor VIII. Blood 91, 538–548 (1998).
Holbrook, J. A., Neu-Yilik, G., Hentze, M. W. & Kulozik, A. E. Nonsense-mediated decay approaches the clinic. Nature Genet. 36, 801–808 (2004).
Thompson, A. R. Structure and function of the factor VIII gene and protein. Semin. Thromb. Hemost. 29, 11–22 (2003).
Vehar, G. A. et al. Structure of the human factor VIII. Nature 312, 337–342 (1984).
Liu, M. L. et al. Hemophilic factor VIII C1- and C2-domain missense mutations and their modeling to the 1.5-Ångstrom human C2-domain crystal structure. Blood. 96, 979–987 (2000).
Jacquemin, M. et al. Point mutations scattered in the FVIII C1 and C2 domains reduce FVIII binding to vWF and are associated to mild/moderate haemophilia A. Thromb, Haemost. (Suppl.), 222 (1999).
Jacquemin, M. et al. A novel cause of mild/moderate hemophilia A: mutations scattered in the factor VIII C1 domain reduce factor VIII binding to von Willebrand factor. Blood 96, 958–965 (2000).
Stoilova-McPhie, S., Villoutreix, B. O., Mertens, K., Kemball-Cook, G. & Holzenburg, A. 3-Dimensional structure of membrane-bound coagulation factor VIII: modelling of the factor VIII heterodimer within a 3-dimensional density map derived by electron microscopy. Blood 99, 1215–1223 (2002). An impressive paper that reports the three-dimensional structure of the F8 protein.
Purohit, V. S. et al. Topology of factor VIII bound to phosphatidylserine-containing model membranes. Biochim. Biophys. Acta 1617, 31–38 (2003).
Purohit, V. S., Ramani, K., Sarkar, S., Kazazian, H. H. Jr & Balasubramanian, S. V. Lower inhibitor development in Hemophilia A mice following administration of recombinant factor VIII-O-phospho-L-serine complex. J. Biol. Chem. 23 February 2005 [epub ahead of print].
Oldenburg, J. et al. De novo factor VIII gene intron 22 inversion in a female carrier presents as a somatic mosaicism. Blood 96, 2905–2906 (2000).
Goodeve, A. C. & Peake, I. R. The molecular basis of Hemophilia A: genotype–phenotype relationships and inhibitor development. Semin. Thromb. Hemostas. 29, 23–30 (2003).
Fakharzadeh, S. S. & Kazazian, H. H. Jr. Correlation between factor VIII genotype and inhibitor development in hemophilia A. Semin. Thromb. Hemost. 26, 167–171 (2000).
Fay, P. J. & Jenkins, P. V. Mutating factor FVIII: lesions from structure to function. Blood Rev. 19, 15–27 (2005).
Saenko, E. L., Ananyeva, N. M., Shima, M., Hauser, C. A. E. & Pipe, S. W. The future of recombinant coagulation factors. J. Thromb. Haemost. 1, 922–930 (2003).
Bolton-Maggs, P. H. & Pasi, K. J. Haemophilias A and B. Lancet. 361, 1801–1809 (2003).
Nathwani, A. C., Davidoff, A. M. & Tuddenham, E. G. D. Prospects for gene therapy of haemophilia. Haemophilia 10, 309–318 (2004).
Gruppo, R. A., Brown, D., Wilkes, M. M. & Navickis, R. J. Meta-analytic evidence of increased breakthrough bleeding during prophylaxis with B-domain deleted factor VIII. Haemophilia 10, 747–750 (2004).
Gruppo, R. A., Brown, D., Wilkes, M. M. & Navickis, R. J. Increased breakthrough bleeding during prophylaxis with B-domain deleted factor VIII — a robust meta-analytic finding. Haemophilia 10, 449–451 (2004).
Kessler, C. M. et al. B-domain deleted recombinant factor VIII preparations are bioequivalent to a monoclonal antibody purified plasma-derived factor VIII concentrate: a randomized, three-way crossover study. Haemophilia 11, 84–91 (2005).
Courter, S. G. & Bedrosian, C. L. Clinical evaluation of B-domain deleted recombinant factor VIII in previously untreated patients. Semin. Hematol. 38, 52–59 (2001).
Swaroop, M., Moussalli, M., Pipe, S. W. & Kaufman, R. J. Mutagenesis of a potential immunoglobulin-binding protein-binding site enhances secretion of coagulation factor VIII. J. Biol. Chem. 272, 24121–24124 (1997).
Miao, H. Z. et al. Bioengineering of coagulation factor VIII for improved secretion. Blood 103, 3412–3419 (2004).
Pipe, S. W. & Kaufmann, R. J. Characterization of a genetically engineered inactivation-resistant coagulation factor VIIIa. Proc. Natl Acad. Sci. USA 94, 11851–11856 (1997).
Thornburg, C. D. et al. Inactivation-resistant factor VIII provides superior hemostasis to wild-type factor VIII in a murine hemophilia A model without increased thrombogenicity. Blood 102, 299 (2003).
Gale, A. J., Pellequer, J. L. & Griffin, J. H. A novel engineered interdomain disulfide bond stabilizes human blood coagulation factor VIIIa. J. Thromb. Haemost. 1, OC094 (2003).
Barrow, R. T., Healey, J. F., Gailani, D., Scandella, D., & Lollar, P. Reduction of the antigenicity of factor VIII toward complex inhibitory antibody plasmas using multiply-substituted hybrid human/porcine factor VIII molecules. Blood 95, 564–568 (2000).
Villard, S. et al. Low molecular weight peptides restore the procoagulant activity of factor VIII in the presence of the potent inhibitor antibody ESH8. J. Biol. Chem. 277, 27232–27239 (2002).
Villard, S. et al. Peptide decoys selected by phage display block in vitro and in vivo activity of a human anti-FVIII inhibitor. Blood 102, 949–952 (2003).
Scandella, D. et al. Some factor VIII inhibitor antibodies recognize a common epitope corresponding to C2 domain amino acids 2248 through 2312, which overlap a phospholipid-binding site. Blood 86, 1811–1819 (1995).
Dazzi, F. et al. High incidence of anti-FVIII antibodies against non-coagulant epitopes in haemophilia A patients: a possible role for the half-life of transfused FVIII. Br. J. Haematol. 93, 688–693 (1996).
Lacroix-Desmazes, S. et al. Antibodies with hydrolytic activity towards factor VIII in patients with hemophilia A. J. Immunol. Methods 269, 251–256 (2002).
Brackmann, H. H. & Gormsen, J. Massive factor-VIII infusion in haemophiliac with factor-VIII inhibitor, high responder. Lancet 2, 933 (1977). This case report was the basis of the immune-tolerance therapy.
Moreau, A. et al. Antibodies to the FVIII light chain that neutralize FVIII procoagulant activity are present in plasma of nonresponder patients with severe hemophilia A and in normal polyclonal human IgG. Blood 95, 3435–3441 (2000).
Gilles, J. G., Vanzieleghem, B. & Saint-Remy, J. M. Factor VIII inhibitors. Natural autoantibodies and anti-idiotypes. Semin. Thromb. Hemost. 26, 151–155 (2000).
Reddy, P. S. et al. Sustained human factor VIII expression in hemophilia A mice following systemic delivery of a gutless adenoviral vector. Mol. Ther. 5, 63–73 (2002).
Connelly, S. et al. Complete short-term correction of canine hemophilia A by in vivo gene therapy. Blood 88, 3846–3853 (1996).
Brown, B. D. et al. Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood 103, 804–810 (2004).
Chao, H., Mao, L., Bruce, A. T. & Walsh, C. E. Sustained expression of human factor VIII in mice using a parvovirus-based vector. Blood 95, 1594–1599 (2000).
Chua, M. K. L., Collen, D. & VandenDriessche, T. Clinical gene transfer studies for Hemophilia A. Sem. Thromb. Hemost. 30, 249–256 (2004).
Powell, J. S. et al. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood 102, 2038–2045 (2003).
Stein, C. S. et al. In vivo treatment of hemophilia A and mucopolysaccharidosis type VII using nonprimate lentiviral vectors. Mol. Ther. 3, 850–856 (2001).
Roth, D. A., Tawa, N. E. Jr, O'Brien, J. M., Treco, D. A. & Selden, R. F. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N. Engl. J. Med. 344, 1735–1742 (2001).
Roth, D. A. et al. Implantation of non-viral ex vivo genetically modified autologous dermal fibroblasts that express B-domain deleted human factor VIII in 12 severe hemophilia A study subjects. Blood Abstr. 100, 116 (2002).
Elder, B., Lakich, D. & Gitschier, J. Sequence of the murine factor VIII cDNA. Genomics 16, 374–379 (1993).
Watzka, M., Geisen, C., Seifried, E. & Oldenburg, J. Sequence of the rat factor VIII cDNA. Thromb. Haemost. 91, 38–42 (2004).
Myles, T., Yun, T. H. & Leung, L. K. Structural requirements for the activation of human factor VIII by thrombin. Blood 100, 2820–2826 (2002).
Mannucci, P. M. & Tuddenham, E. G. The Hemophilias — from royal genes to gene therapy. N. Engl. J. Med. 334, 1773–1779 (2001).
Lenting, P. J., van Mourik, J. A. & Mertens, K. The life cycle of coagulation factor VIII in view of its structure and function. Blood 92, 3983–3996 (1998).
Lakich, D., Kazazian, H. H. Jr, Antonarakis, S. E. & Gitschier, J. Inversions disrupting the factor VIII gene are a common cause of severe haemophilia A. Nature Genet. 5, 236–241 (1993). The paper identifies the intron-22 inversion in the F8 gene as being caused by homologous recombination of intron-22 sequences with repetitive sequences outside the F8 gene.
Acknowledgements
This work of H.H.B., J.G., J.O., R.S. and W.S. was supported in part by a grant from the German Human Genome Project (DHGP) to the 'Genotype–Phenotype Correlation in Haemophilia A' consortium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez
OMIM
FURTHER INFORMATION
International Society on Thrombosis and Haemostasis web site
Glossary
- ANTI-IDIOTYPIC ANTIBODIES
-
Antibodies that bind to the specific antigen-binding sites of other antibodies.
- CD4+ CELLS
-
A subset of white blood cells that identify, attack and destroy infection and cancerous cells.
- CHRONIC SYNOVITIS
-
The concurrent non-use of muscles around a joint that is subject to repeated bleeding leads to a periarticular muscular atrophy with loss of dynamic support, which predisposes the joint to further bleeding.
- THROMBOPHILIA
-
Abnormal haemostasis parameters, as assessed in the laboratory, that are associated with an increased risk of blood clotting in veins or arteries.
- CERULOPLASMIN
-
A plasma metalloprotein that binds most copper ions in the plasma. It is involved in the peroxidation of Fe2+ transferrin to form Fe3+ transferrin. Ceruloplasmin, F5 and F8 have a common evolutionary origin. Loss of ceruloplasmin activity leads to diabetes mellitus, retinal degeneration and dementia.
- ONE-STAGE CLOTTING ASSAY
-
Plasma from a patient is mixed with plasma that is known to be deficient in F8, and the time of formation of a fibrin clot is measured. The assay therefore compares the activity of the blood coagulation cascade in the patient's plasma with the normal activity.
- CHROMOGENIC ASSAY
-
Diluted patient plasma is mixed with thrombin, F10 and activated F9 reagents. Activated F9 hydrolyses the chromogenic substrate S–2,765 and releases a chromophore. The colour intensity released by the chromophore is proportional to F8 activity in the sample.
- ALLOANTIBODY
-
Usually refers to an antibody that is raised naturally against foreign tissues from a member of the same species; in the case of haemophilia it describes the formation of antibodies against the therapeutic F8 protein. As the recombinant protein is of human origin it should be recognized as 'self', but it is not.
- TRANSAMINASE
-
A class of enzymes that causes transamination; that is, the transfer of an amino group. Elevated levels of transminase indicate various hepatic disorders.
- OMENTUM
-
A sheet of fat that is covered by peritoneum. The greater omentum is attached to the bottom edge of the stomach and hangs down in front of the intestine. Its other edge is attached to the transverse colon. The lesser omentum is attached to the top edge of the stomach and extends to the lower surface of the liver.
- RANDOMIZED THREE-WAY CROSS-OVER STUDY
-
Three groups of patients are formed by the random distribution of probands. Participants in one group receive one intervention for a period of time, then switch over to a second intervention and then to a third intervention (participants in the second group start with the second intervention and switch to the third, then the first, and so on). The result is that all groups will be treated with all three protocols (so, the term 'cross-over' is used).
- HAEMOSTASIS
-
A balanced interaction of blood cells, vasculature, plasma proteins and low molecular weight substances. Perfect homeostasis means absence of bleeding and of thrombosis.
Rights and permissions
About this article
Cite this article
Graw, J., Brackmann, HH., Oldenburg, J. et al. Haemophilia A: from mutation analysis to new therapies. Nat Rev Genet 6, 488–501 (2005). https://doi.org/10.1038/nrg1617
Issue Date:
DOI: https://doi.org/10.1038/nrg1617
This article is cited by
-
Abnormal frequency of the memory B cell subsets and plasmablasts in patients with congenital severe hemophilia A: correlation with “Inhibitor” formation
Blood Research (2024)
-
African Americans and European Americans exhibit distinct gene expression patterns across tissues and tumors associated with immunologic functions and environmental exposures
Scientific Reports (2021)
-
Rapid genotyping of F8 intron 22 inversion by nested PCR based on long-distance PCR
Journal of Thrombosis and Thrombolysis (2020)
-
Advances in gene therapy for hemophilia
Journal of Biosciences (2020)
-
Restoration of FVIII expression by targeted gene insertion in the FVIII locus in hemophilia A patient-derived iPSCs
Experimental & Molecular Medicine (2019)