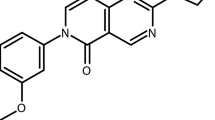
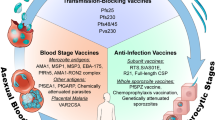
Abstract
Almost all the drugs that are widely used today against Plasmodium spp., the causative agent of malaria, target the asexual blood stages of the parasite. Widespread drug resistance severely restricts our ability to control malaria and makes it necessary to seek novel antimalarial compounds. Here, we advocate the development of true causal chemoprophylactic drugs that will fully inhibit the obligate short-lived hepatic forms that precede blood infections. Such drugs will prevent pathology and interrupt transmission, and could therefore have an important role in the control of malaria and its eventual eradication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
White, N. J. et al. Averting a malaria disaster. Lancet 353, 1965–1967 (1999).
Newton, P. N. & White, N. J. Malaria: new developments in treatment and prevention. Ann. Rev. Med. 50, 179–192 (1999).
White, N. J. Antimalarial drug resistance and combination chemotherapy. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 354, 739–749 (1999).
Noedl, H. et al. Evidence of artemisinin-resistant malaria in western Cambodia. New Engl. J. Med. 359, 2619–2620 (2008).
Baird, J. K. et al. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 44, 547–552 (1991).
Rieckmann, K. H., Davis, D. R. & Hutton, D. C. Plasmodium vivax resistance to chloroquine? Lancet 334, 1183–1184 (1989).
Bunnag, D. et al. High dose of primaquine in primaquine resistant vivax malaria. Trans. Royal Soc. Trop. Med. Hyg. 88, 218–219 (1994).
Luzzi, G. A., Warrell, D. A., Barnes, A. J. & Dunbar, E. M. Treatment of primaquine-resistant Plasmodium vivax malaria. Lancet 340, 310 (1992).
Mendis, K. N., Sina, B. J., Marchesini, P. & Carter, R. The neglected burden of Plasmodium vivax malaria. Am. J. Trop.Med. Hyg. 64, 97–106 (2001).
Rogerson, S. J. & Carter, R. Severe vivax malaria: newly recognised or rediscovered? PLoS Med. 5, e136 (2008).
Tjitra, E. et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 5, e128 (2008).
Shortt, H. E. & Garnham, P. C. C. Pre-erythrocytic stage in mammalian malaria parasites. Nature 161, 126 (1948).
Bray, R. S. Studies on the exo-erythrocytic cycle in the genus Plasmodium (H. K. Lewis & Co., London, 1957).
Krotoski, W. A. et al. Relapses in primate malaria: discovery of two populations of exoerythrocytic stages. Preliminary note. BMJ 280, 153–154 (1980).
Garnham, P. C. C. Malaria Parasites and Other Haemosporidia (Blackwell Scientific Publications, Oxford, 1966).
Cox, F. E. G. in Malaria: Principles and Practice of Malariology (eds. Wernsdorfer, W. H. & McGregor, I.) 1503–1543 (Churchill Livingstone, London, 1988).
Landau, I. & Boulard, Y. in Rodent Malaria (eds. Killick-Kendrick, R. & Peters, W.) 53–84 (Academic Press, London, New York, San Francisco, 1978).
Lupascu, G. et al. The late primary exo-erythrocytic stages of Plasmodium malariae. Trans. Royal Soc.Trop. Med. Hyg. 61, 482–489 (1967).
Boyd, M. F. & Kitchen, S. F. Observations on induced falciparum malaria. Am. J. Trop. Med. 17, 213–235 (1937).
Boyd, M. F. & Stratman-Thomas, W. K. Studies on benign tertian malaria. 7. Some observations on inoculation and onset. Am. J. Hyg. 20, 488–495 (1934).
Bray, R. S. The exoerythrocytic phase of malaria parasites. Int. Rev. Trop. Med. 2, 41–74 (1963).
Fairley, N. H. Sidelights on malaria in man obtained by subinoculation experiments. Trans. Royal Soc. Trop. Med. Hyg. 40, 621–676 (1947).
Bray, R. S. The tissue phase of malaria parasites. J. Trop. Med. Hyg. 57, 41–45 (1954).
Vanderberg, J. P. Asynchronous maturation of Plasmodium berghei exo-erythrocytic forms in rats. Trans. Royal Soc. Trop. Med. Hyg. 76, 251–252 (1982).
Kitchen, S. F. in Malariology — A Comprehensive Survey of All Aspects of This Group of Diseases From a Global Standpoint (ed. Boyd, M. F.) 966–994 (W. B. Saunders Company, Philadelphia and London, 1949).
Haynes, J. D., Diggs, C. L., Hines, F. A. & Desjardins, R. E. Culture of human malaria parasites Plasmodium falciparum. Nature 263, 767–769 (1976).
Trager, W. & Jensen, J. B. Human malaria parasites in continuous culture. Science 193, 673–675 (1976).
Al-Olayan, E. M., Beetsma, A. L., Butcher, G. A., Sinden, R. E. & Hurd, H. Complete development of mosquito phases of the malaria parasite in vitro. Science 295, 677–679 (2002).
Porter-Kelley, J. M. et al. Plasmodium yoelii: axenic development of the parasite mosquito stages. Exp. Parasitol. 112, 99–108 (2006).
Luke, T. C. & Hoffman, S. L. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J. Exp. Biol. 206, 3803–3808 (2003).
Coatney, G. R. & Cooper, W. C. Symposium on exoerythrocytic forms of malarial parasites. III. The chemotherapy of malaria in relation to our knowledge of exoerythrocytic forms. J. Parasitol. 34, 275–289 (1948).
Landau, I. Description of Plasmodium chabaudi n. sp., parasite of African rodents. C. R. Hebd. Séances Acad. Sci. 260, 3758–3761 (1965) (in French).
Landau, I. & Chabaud, A. G. Natural infection by 2 plasmodia of the rodent Thamnomys rutilans in the Central African Republic. C. R. Hebd. Séances Acad. Sci. 261, 230–232 (1965) (in French).
Rodhain, J. Plasmodium vinckei n. sp.; second plasmodium parasite of wild rodents at Katange. Ann. Soc. Belge Méd. Trop. 32, 275–279 (1952) (in French).
Vincke, I. H. & Lips, M. A. H. Un nouveau plasmodium d'un rongeur sauvage du Congon, Plasmodium berghei n. sp. Ann. Soc. Belge Méd. Trop. 28, 97–104 (1948) (in French).
Yoeli, M. & Wall, W. J. Complete sporogonic development of Plasmodium berghei in experimentally infected Anopheles spp. Nature 168, 1078–1080 (1951).
Peters, W. Chemotherapy and drug resistance in malaria (Academic Press, London and New York, 1970).
Schmidt, L. H. Plasmodium cynomolgi infections in the rhesus monkey. Background studies. Am. J. Trop. Med. Hyg. 31, 609–611 (1982).
Schmidt, L. H. et al. Antimalarial activities and subacute toxicity of RC-12, a 4-amino-substituted pyrocatechol. Antimicrob. Agents Chemother. 28, 612–625 (1985).
Herrera, S., Perlaza, B. L., Bonelo, A. & Arévalo-Herrera, M. Aotus monkeys: their great value for anti-malaria vaccines and drug testing. Int. J. Parasitol. 32, 1625–1635 (2002).
Collins, W. E. Nonhuman primate models. II. Infection of Saimiri and Aotus monkeys with Plasmodium vivax. Methods Mol. Med. 72, 85–92 (2002).
Collins, W. E. Nonhuman primate models. I. Nonhuman primate host-parasite combinations. Methods Mol. Med. 72, 77–84 (2002).
Badell, E. et al. Human malaria in immunocompromised mice: an in vivo model to study defense mechanisms against Plasmodium falciparum. J. Exp. Med. 192, 1653–1660 (2000).
Moreno, A., Badell, E., Van Rooijen, N. & Druilhe, P. Human malaria in immunocompromised mice: new in vivo model for chemotherapy studies. Antimicrob. Agents Chemother. 45, 1847–1853 (2001).
Moreno, A. et al. The course of infections and pathology in immunomodulated NOD/LtSz-SCID mice inoculated with Plasmodium falciparum laboratory lines and clinical isolates. Int. J. Parasitol. 36, 361–369 (2006).
Moreno, A., Perignon, J. L., Morosan, S., Mazier, D. & Benito, A. Plasmodium falciparum-infected mice: more than a tour de force. Trends Parasitol. 23, 254–259 (2007).
Grompe, M. Mouse liver goes human: a new tool in experimental hepatology. Hepatology 33, 1005–1006 (2001).
Kneteman, N. M. & Mercer, D. F. Mice with chimeric human livers: who says supermodels have to be tall? Hepatology 41, 703–706 (2005).
Morosan, S. et al. Liver-stage development of Plasmodium falciparum, in a humanized mouse model. J. Infect. Dis. 193, 996–1004 (2006).
Sacci, J. B. Jr et al. Plasmodium falciparum infection and exoerythrocytic development in mice with chimeric human livers. Int. J. Parasitol. 36, 353–360 (2006).
Azuma, H. et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nature Biotech. 25, 903–910 (2007).
Hollingdale, M. R., Leef, J. L., McCullough, M. & Beaudoin, R. L. In vitro cultivation of the exoerythrocytic stage of Plasmodium berghei from sporozoites. Science 213, 1021–1022 (1981).
Hollingdale, M. R., Leland, P. & Schwartz, A. L. In vitro cultivation of the exoerythrocytic stage of Plasmodium berghei in a hepatoma cell line. Am. J. Trop. Med. Hyg. 32, 682–684 (1983).
Lambiotte, M., Landau, I., Thierry, N. & Miltgen, F. Development of schizonts in cultured hepatocytes of adult rats after in vitro infection with Plasmodium yoelii sporoazoites. C. R. Hebd. Séances Acad. Sci. 293, 431–433 (1981) (in French).
Pirson, P. Culture of the exoerythrocytic liver stages of Plasmodium berghei sporozoites in rat hepatocytes. Trans. Royal Soc. Trop. Med. Hyg. 76, 422 (1982).
Mazier, D. et al. In vitro infection of adult Thamnomys hepatocytes by sporozoites of Plasmodium yoelii: development of schizonts and release of infective merozoites. Ann. Parasitol. Hum. Comp. 57, 99–100 (1982) (in French).
Rénia, L. et al. A malaria heat-shock-like determinant expressed on the infected hepatocyte surface is the target of antibody-dependent cell-mediated cytotoxic mechanisms by nonparenchymal liver cells. Eur. J. Immunol. 20, 1445–1449 (1990).
Mazier, D. et al. Complete development of hepatic stages of Plasmodium falciparum in vitro. Science 227, 440–442 (1985).
Mazier, D. et al. Cultivation of the liver forms of Plasmodium vivax in human hepatocytes. Nature 307, 367–369 (1984).
Mazier, D. et al. Plasmodium ovale: in vitro development of hepatic stages. Exp. Parasitol. 64, 393–400 (1987).
Smith, J. E., Meis, J. F., Ponnudurai, T., Verhave, J. P. & Moshage, H. J. In vitro culture of exoerythrocytic form of Plasmodium falciparum in adult human hepatocytes. Lancet 2, 757–758 (1984).
Millet, P. G., Collins, W. E., Fisk, T. L. & Nguyen-Dinh, P. In vitro cultivation of exoerythrocytic stages of the human malaria parasite Plasmodium malariae. Am. J. Trop. Med. Hyg. 38, 470–473 (1988).
Millet, P. G. et al. In vitro cultivation of Plasmodium cynomolgi bastianelli in hepatocytes of the Macaca rhesus. Ann. Parasitol. Hum. Comp. 62, 5–7 (1987) (in French).
Millet, P. G., Fisk, T. L., Collins, W. E., Broderson, J. R. & Nguyen-Dinh, P. Cultivation of exoerythrocytic stages of Plasmodium cynomolgi, P. knowlesi, P. coatneyi, and P. inui in Macaca mulatta hepatocytes. Am. J. Trop. Med. Hyg. 39, 529–534 (1988).
Karnasuta, C. et al. Complete development of the liver stage of Plasmodium falciparum in a human hepatoma cell line. Am. J. Trop. Med. Hyg. 53, 607–611 (1995).
Sattabongkot, J. et al. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am. J. Trop. Med. Hyg. 74, 708–715 (2006).
Prudencio, M., Rodriguez, A. & Mota, M. M. The silent path to thousands of merozoites: the Plasmodium liver stage. Nature Rev. Microbiol. 4, 849–856 (2006).
Calvo-Calle, J. M., Moreno, A., Eling, W. M. C. & Nardin, E. H. In vitro development of infectious liver stages of P. yoelii and P. berghei malaria in human cell lines. Exp. Parasitol. 79, 362–373 (1994).
Hollingdale, M. R. Malaria and the liver. Hepatology 5, 327–335 (1985).
Silvie, O. et al. Expression of human CD81 differently affects host cell susceptibility to malaria sporozoites depending on the Plasmodium species. Cell. Microbiol. 8, 1134–1146 (2006).
Hollingdale, M. R., Collins, W. E., Campbell, C. C. & Schwartz, A. L. In vitro culture of two populations (dividing and nondividing) of exoerythrocytic parasites of Plasmodium vivax. Am. J. Trop. Med. Hyg. 34, 216–222 (1985).
Hollingdale, M. R., Collins, W. E. & Campbell, C. C. In vitro culture of exoerythrocytic parasites of the North Korean strain of Plasmodium vivax in hepatoma cells. Am. J. Trop. Med. Hyg. 35, 275–276 (1986).
Silvie, O. et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J. Biol. Chem. 279, 9490–9406 (2004).
Meis, J. F. et al. Infection of cryopreserved adult human hepatocytes with Plasmodium falciparum sporozoites. Cell Biol. Int. Rep. 9, 976 (1985).
Rénia, L. et al. Malaria sporozoite penetration. A new approach by double staining. J. Immunol. Methods 112, 201–205 (1988).
Mazier, D. et al. Effect of antibodies to recombinant and synthetic peptides on P. falciparum sporozoites in vitro. Science 231, 156–159 (1986).
Gego, A. et al. New approach for high-throughput screening of drug activity on Plasmodium liver stages. Antimicrob. Agents Chemother. 50, 1586–1589 (2006).
Rodrigues, C. D. et al. Host scavenger receptor SR-BI plays a dual role in the establishment of malaria parasite liver infection. Cell Host Microbe 4, 271–282 (2008).
Prudencio, M., Rodrigues, C. D., Ataide, R. & Mota, M. M. Dissecting in vitro host cell infection by Plasmodium sporozoites using flow cytometry. Cell. Microbiol. 10, 218–224 (2008).
Jung, M. et al. Effects of hepatocellular iron imbalance on nitric oxide and reactive oxygen intermediates production in a model of sepsis. J. Hepatol. 33, 387–394 (2000).
Yalaoui, S. et al. Hepatocyte permissiveness to Plasmodium infection is conveyed by a short and structurally conserved region of the CD81 large extracellular domain. PLoS Pathogen 4, e1000010 (2008).
Most, H., Herman, R. H. & Schoenfeld, C. Chemotherapy of sporozoite- and blood-induced Plasmodium berghei infections with selected antimalarial agents. Am. J. Trop. Med. Hyg. 16, 572–575 (1967).
Fink, E. Assessment of causal prophylactic activity in Plasmodium berghei yoelii and its value for the development of new antimalarial drugs. Bull. World Health Organ. 50, 213–222 (1974).
Hulier, E. et al. A method for the quantitative assessment of malaria parasite development in organs of the mammalian host. Mol. Biochem. Parasitol. 77, 127–135 (1996).
Briones, M. R. S., Tsuji, M. & Nussenzweig, V. The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes. Mol. Biochem. Parasitol. 77, 7–17 (1996).
Bruña-Romero, O. et al. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31, 1499–1502 (2001).
Witney, A. A. et al. Determining liver stage parasite burden by real time quantitative PCR as a method for evaluating pre-erythrocytic malaria vaccine efficacy. Mol. Biochem. Parasitol. 118, 233–245 (2001).
Snounou, G., Grüner, A. C., Müller-Graf, C. D., Mazier, D. & Rénia, L. The Plasmodium sporozoite survives RTS,S vaccination. Trends Parasitol. 21, 456–461 (2005).
Carraz, M. et al. A plant-derived morphinan as a novel lead compound active against malaria liver stages. PLoS Medicine 3, e513 (2006).
Grüner, A. C. et al. Insights into the P. y. yoelii hepatic stage transcriptome reveal complex transcriptional patterns. Mol. Biochem. Parasitol. 142, 184–192 (2005).
Sacci, J. B. Jr, Aguiar, J. C., Lau, A. O. T. & Hoffman, S. L. Laser capture microdissection and molecular analysis of Plasmodium yoelii liver-stage parasites. Mol. Biochem. Parasitol. 119, 285–289 (2002).
Semblat, J.-P. et al. Laser capture microdissection of Plasmodium falciparum liver stages for mRNA analysis. Mol. Biochem. Parasitol. 121, 179–183 (2002).
Siau, A. et al. Temperature shift and host cell contact up-regulate sporozoite expression of Plasmodium falciparum genes involved in hepatocyte infection. PLoS Pathogen 4, e1000121 (2008).
Tarun, A. S. et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc. Natl Acad. Sci. USA 105, 305–310 (2008).
Cunha-Rodrigues, M., Prudencio, M., Mota, M. M. & Haas, W. Antimalarial drugs — host targets (re)visited. Biotechnol. J. 1, 321–332 (2006).
Mahmoudi, N. et al. New active drugs against liver stages of Plasmodium predicted by molecular topology. Antimicrob. Agents Chemother. 52, 1215–1220 (2008).
Doerig, C. & Meijer, L. Antimalarial drug discovery: targeting protein kinases. Exp. Opin. Ther. Targets 11, 279–290 (2007).
Edgcomb, J. H., Arnold, J. D., Yount, E. H. Jr, Alving, A. S. & Eichelberger, L. Primaquine, SN 13272, a new curative agent in vivax malaria: a preliminary report. J. Natl Malaria Soc. 9, 285–292 (1950).
Hill, D. R. et al. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am. J. Trop. Med. Hyg. 75, 402–415 (2006).
Baird, J. K., Fryauff, D. J. & Hoffman, S. L. Primaquine for prevention of malaria in travelers. Clin. Infect. Dis. 37, 1659–1667 (2003).
Peters, W. The evolution of tafenoquine — antimalarial for a new millennium? J. Royal Soc. Med. 92, 345–352 (1999).
Brueckner, R. P., Coster, T., Wesche, D. L., Shmuklarsky, M. J. & Schuster, B. G. Prophylaxis of Plasmodium falciparum infection in a human challenge model with WR 238605, a new 8-aminoquinoline antimalarial. Antimicrob. Agents Chemother. 42, 1293–1294 (1998).
Dutta, G. P., Puri, S. K., Bhaduri, A. P. & Seth, M. Radical curative activity of a new 8-aminoquinoline derivative (CDRI 80/53) against Plasmodium cynomolgi B in monkeys. Am. J. Trop. Med. Hyg. 41, 635–637 (1989).
Krudsood, S. et al. Safety and tolerability of elubaquine (bulaquine, CDRI 80/53) for treatment of Plasmodium vivax malaria in Thailand. Korean J. Parasitol. 44, 221–228 (2006).
Valecha, N. et al. Comparative antirelapse efficacy of CDRI compound 90/53 (Bulaquine) vs primaquine in double blind clinical trials. Curr. Sci. 80, 561–563 (2001).
Alving, A. S. et al. Potentiation of the curative action of primaquine in vivax malaria by quinine and chloroquine. J. Lab. Clin. Med. 46, 301–306 (1955).
Schmidt, L. H., Fradkin, R., Vaughan, D. & Rasco, J. Radical cure of infections with Plasmodium cynomolgi: a function of total 8-aminoquinoline dose. Am. J. Trop. Med. Hyg. 26, 1116–1128 (1977).
Alving, A. S. et al. Korean vivax malaria. II. Curative treatment with pamaquine and primaquine. Am. J. Trop. Med. Hyg. 2, 970–976 (1953).
Arnold, J. D., Alving, A. S. & Clayman, C. B. Induced primaquine resistance in vivax malaria. Trans. Royal Soc. Trop. Med. Hyg. 55, 345–350 (1961).
Singh, N. & Puri, S. K. Causal prophylactic activity of antihistaminic agents against Plasmodium yoelii nigeriensis infection in Swiss mice. Acta Trop. 69, 255–260 (1998).
Singh, N. & Puri, S. K. Inhibition of the development of the hepatic stages of Plasmodium yoelii nigeriensis by antihistaminic agents. Ann. Trop. Med. Parasitol. 93, 419–422 (1999).
Zhang, Q. et al. Unambiguous synthesis and prophylactic antimalarial activities of imidazolidinedione derivatives. J. Med. Chem. 48, 6472–6481 (2005).
Coppi, A. et al. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe 2, 316–327 (2007).
Bosch, J. et al. Aldolase provides an unusual binding site for thrombospondin-related anonymous protein in the invasion machinery of the malaria parasite. Proc. Natl Acad. Sci. USA 104, 7015–7020 (2007).
Coppi, A., Pinzon-Ortiz, C., Hutter, C. & Sinnis, P. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J. Exp. Med. 201, 27–33 (2005).
Talati, S. M., Latham, M. R., Moore, E. G., Hargreaves, G. W. & DeWitt Blanton, C. Jr. Synthesis of potential antimalarials: primaquine analogs. J. Pharm. Sci. 59, 491–495 (1970).
Li, J. et al. Plasmodium berghei: quantitation of in vitro effects of antimalarial drugs on exoerythrocytic development by a ribosomal RNA probe. Exp. Parasitol. 72, 450–458 (1991).
Powell, R. D. & Brewer, G. J. Effects of pyrimethamine, chlorguanide, and primaquine against exoerythrocytic forms of a strain of chloroquine-resistant Plasmodium falciparum from Thailand. Am. J. Trop. Med. Hyg. 16, 693–698 (1967).
Nduati, E. et al. Effect of folate derivatives on the activity of antifolate drugs used against malaria and cancer. Parasitol. Res. 102, 1227–1234 (2008).
Gregory, K. G. & Peters, W. The chemotherapy of rodent malaria. IX. Causal prophylaxis. I. A method for demonstrating drug action on exo-erythrocytic stages. Ann. Trop. Med. Parasitol. 64, 15–24 (1970).
Hollingdale, M. R., McCann, P. P. & Sjoerdsma, A. Plasmodium berghei: inhibitors of ornithine decarboxylase block exoerythrocytic schizogony. Exp. Parasitol. 60, 111–117 (1985).
Gantt, S. M. et al. Proteasome inhibitors block development of Plasmodium spp. Antimicrob. Agents Chemother. 42, 2731–2738 (1998).
Davies, C. S., Pudney, M., Nicholas, J. C. & Sinden, R. E. The novel hydroxynaphthoquinone 566C80 inhibits the development of liver stages of Plasmodium berghei cultured in vitro. Parasitology 106, 1–6 (1993).
Andersen, S. L. et al. Efficacy of azithromycin as a causal prophylactic agent against murine malaria. Antimicrob. Agents Chemother. 38, 1862–1863 (1994).
Marussig, M. S. et al. Activity of doxycycline against preerythrocytic malaria. J. Infect. Dis. 168, 1603–1604 (1993).
Mahmoudi, N. et al. In vitro activities of 25 quinolones and fluoroquinolones against liver and blood stage Plasmodium spp. Antimicrob. Agents Chemother. 47, 2636–2639 (2003).
Yu, M. et al. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe 4, 567–578 (2008).
Vaughan, A. M. et al. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell. Microbiol. 11, 506–520 (2009).
Van De Sand, C. et al. The liver stage of Plasmodium berghei inhibits host cell apoptosis. Mol. Microbiol. 58, 731–742 (2005).
Sturm, A. et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313, 1287–1290 (2006).
Acknowledgements
We are grateful to A. C. Grüner for critical reading of the manuscript and many helpful and pertinent suggestions. D.M., L.R. and G.S. are currently part of an official collaboration between SIgN-/A*-STAR and INSERM (Laboratoire International Associé, INSERM).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information S1 (table)
Compounds with known anti-LS activity (PDF 625 kb)
Related links
Rights and permissions
About this article
Cite this article
Mazier, D., Rénia, L. & Snounou, G. A pre-emptive strike against malaria's stealthy hepatic forms. Nat Rev Drug Discov 8, 854–864 (2009). https://doi.org/10.1038/nrd2960
Issue Date:
DOI: https://doi.org/10.1038/nrd2960
This article is cited by
-
Chemoprotective antimalarials identified through quantitative high-throughput screening of Plasmodium blood and liver stage parasites
Scientific Reports (2021)
-
Human unconventional T cells in Plasmodium falciparum infection
Seminars in Immunopathology (2020)
-
A novel immortalized hepatocyte-like cell line (imHC) supports in vitro liver stage development of the human malarial parasite Plasmodium vivax
Malaria Journal (2018)
-
Towards a Humanized Mouse Model of Liver Stage Malaria Using Ectopic Artificial Livers
Scientific Reports (2017)
-
The Plasmodium PI(4)K inhibitor KDU691 selectively inhibits dihydroartemisinin-pretreated Plasmodium falciparum ring-stage parasites
Scientific Reports (2017)