Key Points
-
The RAS superfamily of GTPases includes five major subfamilies of monomeric guanine nucleotide binding proteins (RAS, RHO, RAB, RAN and ARF), which possess GDP/GTP-binding and intrinsic GTPase activities that enable them to interconvert between biologically active (GTP-bound) and inactive (GDP-bound) conformations.
-
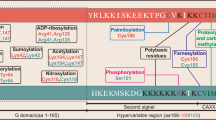
RAS GTPases undergo extensive post-translational modifications, which facilitate their membrane attachment and determine their subcellular localization and function.
-
GDP/GTP exchange is controlled by a complex regulatory network comprising several classes of proteins including GTPase activating proteins (GAPs), guanine nucleotide exchange factors (GEFs) and guanine nucleotide dissociation inhibitors (GDIs).
-
Mutational activation, gene amplification and genetic rearrangements involving RAS GTPases and their regulatory proteins (RPs) are prevalent in a various human malignancies.
-
Pharmacological strategies targeting the RAS superfamily of GTPases include inhibitors of their post-translational modifications (mevalonate pathway, prenylation and post-prenylation inhibitors) and inhibitors of their RPs — RP inhibitors and RP–RAS GTPase interfacial inhibitors.
-
Several lines of evidence indicate synergistic activity of these agents with conventional chemotherapeutics, radiation therapy and other molecularly targeted agents. These agents demonstrate different specificities towards RAS GTPases, with interfacial inhibitors showing the highest specificity, and the prenylation and post-prenylation inhibitors being the most 'unspecific' , capable of targeting several RAS GTPases.
-
Identification of appropriate pharmacodynamic molecular and radiographic end points that can guide dose escalation and assess responses to treatment represent a major challenge for the development of these agents.
Abstract
The involvement of the RAS superfamily of monomeric GTPases in carcinogenesis is increasingly being appreciated. A complex array of post-translational modifications and a highly sophisticated protein network regulate the spatio-temporal activation of these GTPases. Previous attempts to pharmacologically target this family have focused on the development of farnesyltransferase inhibitors, but the performance of such agents in cancer clinical trials has not been as good as hoped. Here, we review emerging druggable targets and novel therapeutic approaches targeting prenylation and post-prenylation modifications and the functional regulation of GDP/GTP exchange as exciting alternatives for anticancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gilman, A. G. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56, 615–649 (1987).
Oldham, W. M., Van Eps, N., Preininger, A. M., Hubbell, W. L. & Hamm, H. E. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nature Struct. Mol. Biol. 9, 772–777 (2006).
Takai, Y., Sasaki, T. & Matozaki, T. Small GTP-binding proteins. Physiol. Rev. 81, 153–208 (2001).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002).
Pfeffer, S. & Aivazian, D. Targeting Rab GTPases to distinct membrane compartments. Nature Rev. Mol. Cell Biol. 5, 886–896 (2004).
Rocks, O., Peyker, A. & Bastiaens, P. I. Spatio-temporal segregation of Ras signals: one ship, three anchors, many harbors. Curr. Opin. Cell Biol. 18, 351–357 (2006).
Quimby, B. B. & Dasso, M. The small GTPase Ran: nterpreting the signs. Curr. Opin, Cell Biol. 15, 338–344 (2003).
D'Souza-Schorey, C. & Chavrier, P. ARF proteins: roles in membrane traffic and beyond. Nature Rev. Mol. Cell Biol. 7, 347–358 (2006).
Manser, E. Small GTPases take the stage. Dev. Cell 3, 323–328 (2002).
Bernards, A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta 1603, 47–82 (2003).
Rossman K. L., Der C. J. & Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nature Rev. Mol. Cell Biol. 6, 167–180 (2005).
DerMardirossian, C. & Bokoch, G. M. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15, 356–363 (2005).
Radhika, V. & Dhanasekaran, N. Transforming G proteins. Oncogene 20, 1607–1614 (2001).
Shaw, R. J. & Cantley, L. C. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441, 424–430 (2006).
Bos, J. L. Ras oncogenes in human cancer: a review. Cancer Res. 49, 4682–4689 (1989).
Downward, J. Targeting RAS signalling pathways in cancer therapy. Nature Rev. Cancer 3, 11–22 (2003).
Daub, H., Specht, K. & Ullrich, A. Strategies to overcome resistance to targeted protein kinase inhibitors. Nature Rev. Drug Discov. 3, 1001–1110 (2004).
Sahai, E. & Marshall, C. J. RHO-GTPases and cancer. Nature Rev. Cancer 2, 133–142 (2002).
Preudhomme, C. Non-random 4p13 rearrangements of the RhoH/TTF gene, encoding a GTP-binding protein, in non-Hodgkin's lymphoma and multiple myeloma. Oncogene 19, 2023–2032 (2000).
Pasqualucci, L. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature 412, 341–346 (2001).
Ridley, A. J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16, 522–529 (2006).
Kleer, C. G. et al. RhoC-GTPase is a novel tissue biomarker associated with biologically aggressive carcinomas of the breast. Breast Cancer Res. Treat. 93, 101–110 (2005).
Kamai, T. et al. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin. Cancer Res. 9, 2632–2641 (2003).
Fidyk, N., Wang, J. B. & Cerione, R. A. Influencing cellular transformation by modulating the rates of GTP hydrolysis by Cdc42. Biochemistry 45, 7750–7762 (2006).
Lin, R., Cerione, R. A. & Manor, D. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J. Biol. Chem. 274, 23633–23641 (1999).
Cheng, K. W. et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nature Med. 10, 1251–1256 (2004).
Croizet-Berger, K., Daumerie, C., Couvreur, M., Courtoy, P. J. & van den Hove, M. F. The endocytic catalysts, Rab5a and Rab7, are tandem regulators of thyroid hormone production. Proc. Natl Acad. Sci. USA 99, 8277–8282 (2002).
He, H. et al. Identification and characterization of nine novel human small GTPases showing variable expressions in liver cancer tissues. Gene Expr. 10, 231–242 (2002).
Hashimoto, S. et al. Requirement for ARF6 in breast cancer invasive activities. Proc. Natl Acad. Sci. USA. 101, 6647–6645 (2004).
Calin, G. A. et al. Familial cancer associated with a polymorphism in ARLTS1. N Engl J Med. 352, 1667–76 (2005).
Zhang, F. L. & Casey, P. J. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65, 241–269 (1996).
Reid, T. S., Terry, K. L., Casey, P. J. & Beese L. S. Crystallographic analysis of CAAX prenyltransferases complexed with substrates defines rules of protein substrate selectivity. J. Mol. Biol. 343, 417–433 (2004).
Ashby, M. N. CaaX converting enzymes. Curr. Opin Lipidol. 9, 99–102 (1998).
Hancock, J. F., Magee, A. I., Childs, J. E. & Marshall, C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57, 1167–1177 (1989).
Hoffman, G. R., Nassar, N. & Cerione, R. A. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell 100, 345–356 (2000).
McTaggart, S. J. Isoprenylated proteins. Cell Mol. Life Sci. 63, 255–267 (2006).
Amor, J. C., Harrison, D. H., Kahn, R. A. & Ringe, D. Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature 372, 704–708 (1994).
Donovan, S., Shannon, K. M. & Bollag, G. GTPase activating proteins: critical regulators of intracellular signaling. Biochim. Biophys. Acta 1602, 23–45 (2002).
Weiss, B., Bollag, G. & Shannon, K. Hyperactive Ras as a therapeutic target in neurofibromatosis type 1. Am. J. Med. Genet. 89, 14–22 (1999).
Crino, P. B., Nathanson, K. L. & Henske, E. P. The tuberous sclerosis complex. N. Engl. J. Med. 355, 1345–1355 (2006). An excellent review on the role of RHEB/mTOR in the pathogenesis of tuberous sclerosis.
Dransart, E., Olofsson, B. & Cherfils, J. RhoGDIs revisited: novel roles in Rho regulation. Traffic 6, 957–966 (2005).
Ivetic, A. & Ridley, A. J. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology 112, 165–176 (2004).
Maeda, M., Matsui, T., Imamura, M., Tsukita, S. & Tsukita, S. Expression level, subcellular distribution and rho-GDI binding affinity of merlin in comparison with Ezrin/Radixin/Moesin proteins. Oncogene 18, 4788–4797 (1999).
Kourlas, P. J. et al. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: evidence for its fusion with MLL in acute myeloid leukemia. Proc. Natl Acad. Sci. USA 97, 2145–2150 (2000).
Kin, Y., Li, G, Shibuya, M. & Maru, Y. The Dbl homology domain of BCR is not a simple spacer in P210BCR-ABL of the Philadelphia chromosome. J. Biol. Chem. 276, 39462–39468 (2001).
Engers, R. et al. TIAM1 mutations in human renal-cell carcinomas. Int. J. Cancer 88, 369–376 (2000).
Wolf, R. M. et al. p190RhoGAP can act to inhibit PDGF-induced gliomas in mice: a putative tumor suppressor encoded on human chromosome 19q13.3. Genes Dev. 17, 476–487 (2003).
Peck, J., Douglas, G., Wu, C. H. & Burbelo, P. D. Human RhoGAP domain-containing proteins: structure, function and evolutionary relationships. FEBS Lett. 528, 27–34 (2002).
Leung T. H. et al. Deleted in liver cancer 2 (DLC2) suppresses cell transformation by means of inhibition of RhoA activity. Proc. Natl Acad. Sci. USA 102, 15207–15212 (2005).
MacKeigan, J. P. et al. Proteomic profiling drug-induced apoptosis in non-small cell lung carcinoma: identification of RS/DJ-1 and RhoGDIα. Cancer Res. 63, 6928–6934 (2003).
Tapper, J. et al. Changes in gene expression during progression of ovarian carcinoma. Cancer Genet. Cytogenet. 128, 1–6 (2001).
Jiang, W. G. et al. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin. Cancer Res. 9, 6432–6440 (2003).
Stein, M. P., Dong, J. & Wandinger-Ness, A. Rab proteins and endocytic trafficking: potential targets for therapeutic intervention. Adv. Drug Deliv. Rev. 55, 1421–1437 (2003).
Randazzo, P. A. & Hirsch, D. S. Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal. 16, 401–413 (2004).
Demierre, M. F., Higgins, P. D., Gruber, S. B., Hawk, E. & Lippman, S. M. Statins and cancer prevention. Nature Rev. Cancer 5, 930–942 (2005).
Mo, H. & Elson, C. E. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp. Biol. Med. (Maywood) 229, 567–585 (2004).
Wong, W. W., Dimitroulakos, J., Minden, M. D. & Penn, L. Z. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16, 508–511 (2002).
Weitz-Schmidt, G. et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature Med. 7, 687–692 (2001).
Rao, S. et al. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc. Natl Acad. Sci. USA. 96, 7797–7802 (1999).
Hindler, K. et al. The role of statins in cancer therapy. Oncologist 11, 306–315 (2006). An overview of the clinical trials evaluating the activity of statins as anticancer agents.
Kawata, S. et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br. J. Cancer 84, 886–891 (2001).
van Beek, E. et al. Nitrogen-containing bisphosphonates inhibit isopentenyl pyrophosphate isomerase/farnesyl pyrophosphate synthase activity with relative potencies corresponding to their antiresorptive potencies in vitro and in vivo. Biochem. Biophys. Res Commun. 255, 491–494 (1999).
Santini, D. et al. Mechanisms of disease: preclinical reports of antineoplastic synergistic action of bisphosphonates. Nature Clin. Pract. Oncol. 3, 325–338 (2006).
Alakangas, A. et al. Alendronate disturbs vesicular trafficking in osteoclasts. Calcif. Tissue Int. 70, 40–47 (2002).
Caraglia, M. et al. Emerging anti-cancer molecular mechanisms of aminobisphosphonates. Endocr. Relat. Cancer 13, 7–26 (2006).
Coxon, F. P. et al. Phosphonocarboxylate inhibitors of Rab geranylgeranyl transferase disrupt the prenylation and membrane localization of Rab proteins in osteoclasts in vitro and in vivo. Bone 37, 349–358 (2005).
Basso, A. D., Kirschmeier, P. & Bishop, W. R. Lipid post-translational modifications. Farnesyl transferase inhibitors. J. Lipid Res. 47, 15–31 (2006).
Kohl, N. E. Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science 260, 1934–1937 (1993).
Law, B. K., Norgaard, P. & Moses, H. L. Farnesyltransferase inhibitor induces rapid growth arrest and blocks p70s6k activation by multiple stimuli. J. Biol. Chem. 275, 10796–10801 (2000).
Whyte, D. B. et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J. Biol. Chem. 272, 14459–14464 (1997).
Lebowitz, P. F., Casey, P. J., Prendergast, G. C. & Thissen, J. A. Farnesyltransferase inhibitors alter the prenylation and growth-stimulating function of RhoB. J. Biol. Chem. 272, 15591–15594 (1997).
Du, W., Lebowitz, P. F. & Prendergast, G. C. Cell growth inhibition by farnesyltransferase inhibitors is mediated by gain of geranylgeranylated RhoB. Mol. Cell Biol. 19, 1831–1840 (1999). One of the most important studies that demonstrate that geranylgeranylated RHOB has antigrowth properties, while farnesylated RHOB can be tumorigenic.
Chen, Z. et al. Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumour growth in nude mice. J. Biol. Chem. 275, 17974–17978 (2000).
Basso, A. D. et al. The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J. Biol. Chem. 280, 31101–31108 (2005).
Tabancay, A. P. et al. Identification of dominant negative mutants of Rheb GTPase and their use to implicate the involvement of human Rheb in the activation of p70S6K. J. Biol. Chem. 278, 39921–39930 (2003).
Lackner, M. R. et al. Chemical genetics identifies Rab geranylgeranyl transferase as an apoptotic target of farnesyl transferase inhibitors. Cancer Cell 7, 325–336 (2005).
Appels, N. M., Beijnen, J. H. & Schellens, J. H. Development of farnesyl transferase inhibitors: a review. Oncologist 10, 565–578 (2005).
Adjei, A. A., Davis, J. N., Erlichman, C., Svingen, P. A. & Kaufmann, S. H. Comparison of potential markers of farnesyltransferase inhibition. Clin. Cancer Res. 6, 2318–2325 (2000). A useful discussion of potential biomarkers of FTase inhibition.
Van Cutsem, E. et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J. Clin. Oncol. 22, 1430–1438 (2004).
Rao, S. et al. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J. Clin. Oncol. 22, 3950–3957 (2004).
McDonald, J. S. et al. A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest Oncology Group (SWOG 9924) study. Invest. New Drugs 23, 485–487 (2005).
Zimmerman, T. M. et al. Dose-ranging pharmacodynamic study of tipifarnib (R115777) in patients with relapsed and refractory hematologic malignancies. J. Clin. Oncol. 22, 4816–4822 (2004).
Lancet, J. E. et al. A Phase II study of the farnesyltransferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood 109, 1387–1394 (2007).
Caraglia, M., Budillon, A., Tagliaferri, P., Marra, M., Abbruzzese, A. & Caponigro, F. Isoprenylation of intracellular proteins as a new target for the therapy of human neoplasms: preclinical and clinical implications. Curr. Drug Targets 6, 301–323 (2005).
Di Paolo, A. et al. Inhibition of protein farnesylation enhances the chemotherapeutic efficacy of the novel geranylgeranyltransferase inhibitor BAL9611 in human colon cancer cells. Br. J. Cancer. 84, 1535–1543 (2001).
Lobell, R. B. et al. Evaluation of farnesyl:protein transferase and geranylgeranyl:protein transferase inhibitor combinations in preclinical models. Cancer Res. 61, 8758–8768 (2001).
Morgan, M. A., Wegner, J., Aydilek, E., Ganser, A. & Reuter, C. W. Synergistic cytotoxic effects in myeloid leukemia cells upon cotreatment with farnesyltransferase and geranylgeranyl transferase-I inhibitors. Leukemia 17, 1508–1520 (2003).
Reid, T. S. et al. Crystallographic analysis reveals that anticancer clinical candidate L-778,123 inhibits protein farnesyltransferase and geranygeranyltransferase-I by different binding modes. Biochemistry 43, 9000–9008 (2004).
Lobell, R. B. et al. Preclinical and clinical pharmacodynamic assessment of L-778,123, a dual inhibitor of farnesyl:protein transferase and geranylgeranyl: protein transferase type-I. Mol. Cancer Ther. 1, 747–758 (2002).
Kelly, J. et al. The prenyltransferase inhibitor AZD3409 has anti-tumour activity in preclinical models of urothelial carcinoma. Proc. Am. Assoc. Cancer Res. 46, 5962 (2005).
Bergo, M. O. et al. Absence of the CAAX endoprotease RCE1: effects on cell growth and transformation. Mol. Cell Biol. 22, 171–181 (2002).
Bergo, M. O. et al. Inactivation of Icmt inhibits transformation by oncogenic K-Ras and B-Raf. J. Clin. Invest. 113, 539–550 (2004). A pivotal paper showing that the inhibition of ICMT activity blocks oncogenic KRAS-induced transformation by decreasing the methylation of KRAS, HRAS and NRAS.
Winter-Vann, A. M. & Casey, P. J. Post-prenylation-processing enzymes as new targets in oncogenesis. Nature Rev. Cancer. 5, 405–412 (2005).
Schlitzer, M., Winter-Vann, A. & Casey, P. J. Non-peptidic, non-prenylic inhibitors of the prenyl protein-specific protease Rce1. Bioorg. Med. Chem. Lett. 11, 425–427 (2001).
Chen, Y. Selective inhibition of ras-transformed cell growth by a novel fatty acid-based chloromethyl ketone designed to target Ras endoprotease. Ann. NY Acad. Sci. 886, 103–108 (1999).
Wnuk, S. F. et al. Anticancer and antiviral effects and inactivation of S-adenosyl-L-homocysteine hydrolase with 5′-carboxaldehydes and oximes synthesized from adenosine and sugar-modified analogues. J. Med. Chem. 40, 1608–1618 (1997).
Winter-Vann, A. M. et al. Targeting Ras signaling through inhibition of carboxyl methylation: an unexpected property of methotrexate. Proc. Natl Acad. Sci. USA 100, 6529–6534 (2003).
Winter-Vann, A. M. et al. A small-molecule inhibitor of isoprenylcysteine carboxyl methyltransferase with antitumor activity in cancer cells. Proc. Natl Acad. Sci. USA 102, 4336–4341 (2005).
Michaelson, D. et al. Postprenylation CAAX processing is required for proper localization of Ras but not Rho GTPases. Mol. Biol. Cell 16, 1606–1616 (2005).
Kramer, K., et al. Isoprenylcysteine carboxyl methyltransferase activity modulates endothelial cell apoptosis. Mol. Biol. Cell 14, 848–857 (2003).
Chiu, V. K. et al. Ras signalling on the endoplasmic reticulum and the Golgi. Nature Cell Biol. 4, 343–350 (2002). An important study that provides evidence that mislocalized farnesylated or geranylgeranylated RAS (specifically NRAS and HRAS) can still signal from other locations besides the plasma membrane.
Leow, J. L., Baron, R., Casey, P. J. & Go, M. L. Quantitative structure–activity relationship (QSAR) of indoloacetamides as inhibitors of human isoprenylcysteine carboxyl methyltransferase. Bioorg. Med. Chem. Lett. 17, 1025–1032 (2006).
Roberts, M. J. et al. Hydrophilic anilinogeranyl diphosphate prenyl analogues are ras function inhibitors. Biochemistry 45, 15862–15872 (2006).
Morgan, M. A. Combining prenylation inhibitors causes synergistic cytotoxicity, apoptosis and disruption of RAS-to-MAP kinase signalling in multiple myeloma cells. Br. J. Haematol. 130, 912–925 (2005).
Mo, H. & Elson, C. E. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp. Biol. Med. (Maywood) 229, 567–585 (2004).
Elson, C. E. et al. Isoprenoid-mediated inhibition of mevalonate synthesis: potential application to cancer. Proc. Soc. Exp. Biol. Med. 221, 294–311 (1999).
Andela, V. B. Synergism of aminobisphosphonates and farnesyl transferase inhibitors on tumour metastasis. Clin. Orthop. Relat. Res. 397, 228–239 (2002).
Budman, D. R. & Calabro, A. Zoledronic acid (Zometa) enhances the cytotoxic effect of gemcitabine and fluvastatin: in vitro isobologram studies with conventional and nonconventional cytotoxic agents. Oncology 70, 147–153 (2006).
Hoover, R. R., Mahon, F. X., Melo, J. V. & Daley, G. Q. Overcoming STI571 resistance with the farnesyl transferase inhibitor SCH66336. Blood 100, 1068–1071 (2002).
Jorgensen, H. G. et al. Lonafarnib reduces the resistance of primitive quiescent CML cells to imatinib mesylate in vitro. Leukemia 19, 1184–1191 (2005).
Moasser, M. M. et al. Farnesyl transferase inhibitors cause enhanced mitotic sensitivity to taxol and epothilones. Proc. Natl Acad. Sci. USA 95, 1369–1374 (1998).
Shi, B. et al. The farnesyl protein transferase inhibitor SCH66336 synergizes with taxanes in vitro and enhances their antitumour activity in vivo. Cancer Chemother. Pharmacol. 46, 387–393 (2000).
Marcus, A. I. et al. Farnesyltransferase inhibitors reverse taxane resistance. Cancer Res. 66, 8838–8846 (2006).
Adjei, A. A., Davis, J. N., Bruzek, L. M., Erlichman, C. & Kaufmann, S. H. Synergy of the protein farnesyltransferase inhibitor SCH66336 and cisplatin in human cancer cell lines. Clin. Cancer Res. 7, 1438–1445 (2001).
Doisneau-Sixou, S. F., Cestac, P., Faye, J. C., Favre, G., Sutherland, R. L. Additive effects of tamoxifen and the farnesyl transferase inhibitor FTI-277 on inhibition of MCF-7 breast cancer cell-cycle progression. Int. J. Cancer 106: 789–798 (2003).
Edamatsu, H., Gau, C. L., Nemoto, T., Guo, L., & Tamanoi, F. Cdk inhibitors, roscovitine and olomoucine, synergize with farnesyltransferase inhibitor (FTI) to induce efficient apoptosis of human cancer cell lines. Oncogene 19, 3059–3068 (2000).
Russo, P., Malacarne, D., Falugi, C., Trombino, S. & O'Connor, P. M. RPR-115135, a farnesyltransferase inhibitor, increases 5-FU cytotoxicity in ten human colon cancer cell lines: role of p53. Int. J. Cancer 100, 266–275 (2002).
Neville-Webbe, H. L., Evans, C. A., Coleman, R. E. & Holen, I. Mechanisms of the synergistic interaction between the bisphosphonate zoledronic acid and the chemotherapy agent paclitaxel in breast cancer cells in vitro. Tumour Biol. 27, 92–103 (2006).
Ullen, A. et al. Additive/synergistic antitumoural effects on prostate cancer cells in vitro following treatment with a combination of docetaxel and zoledronic acid. Acta Oncol. 44, 644–650 (2005).
Melisi, D. et al. Zoledronic acid cooperates with a cyclooxygenase-2 inhibitor and gefitinib in inhibiting breast and prostate cancer. Endocr. Relat. Cancer 12, 1051–1058 (2005).
Segawa, H. et al. Zoledronate synergises with imatinib mesylate to inhibit Ph primary leukaemic cell growth. Br. J. Haematol. 130, 558–560 (2005).
Brunner, T. B., Hahn, S. M., McKenna, W. G. & Bernhard, E. J. Radiation sensitization by inhibition of activated Ras. Strahlenther Onkol. 180, 731–740 (2004).
Brunner, T. B. et al. Farnesyltransferase inhibitors as radiation sensitizers. Int. J. Radiat. Biol. 79, 569–576 (2003).
Martin, N. E. et al. A Phase I trial of the dual farnesyltransferase and geranylgeranyltransferase inhibitor L-778,123 and radiotherapy for locally advanced pancreatic cancer. Clin. Cancer Res. 10, 5447–5454 (2004).
Onodera, Y. et al. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 24, 963–973 (2005)
Tesmer, J. J. Hitting the hot spots of cell signaling cascades. Science. 312, 377–378 (2006). An excellent review that discusses the concept of interfacial inhibition and its potential as a strategy for drug discovery.
Pommier, Y. & Cherfils, J. Interfacial inhibition of macromolecular interactions: nature's paradigm for drug discovery. Trends Pharmacol. Sci. 26, 138–145 (2005).
Zeghouf, M., Guibert, B., Zeeh, J. C. & Cherfils, J. Arf, Sec7 and Brefeldin A: a model towards the therapeutic inhibition of guanine nucleotide-exchange factors. Biochem. Soc. Trans. 33, 1265–1268 (2005).
Zeeh, J. et al. Dual specificity of the interfacial inhibitor brefeldin A for arf proteins and sec7 domains. J. Biol. Chem. 281, 11805–11814 (2006).
Gao, Y., Dickerson, J. B., Guo, F., Zheng, J. & Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl Acad. Sci. USA. 101, 7618–7622 (2004). A seminal paper describing the development of NSC23766 by a structure-based virtual screening of compounds that fit into the GEF-recognition groove centring on Trp56 of RAC1.
Schmidt, S., Diriong, S., Mery, J., Fabbrizio, E. & Debant, A. Identification of the first Rho GEF inhibitor, TRIPα, which targets the RhoA-specific GEF domain of Trio. FEBS Lett. 523, 35–44 (2002).
Cancelas, J. A. et al. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nature Med. 11, 886–891 (2005).
Kato-Stankiewicz, J. et al. Inhibitors of Ras/Raf-1 interaction identified by two-hybrid screening revert Ras-dependent transformation phenotypes in human cancer cells. Proc. Natl Acad. Sci. USA. 99, 14398–14403 (2002).
Fritz, G. & Kaina, B. Rho GTPases: promising cellular targets for novel anticancer drugs. Curr. Cancer Drug Targets 6, 1–14 (2006).
Just, I. et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375, 500–503 (1995).
Pille, J. Y. et al. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol. Ther. 11, 267–274 (2005).
Zhang, B. Rho GDP dissociation inhibitors as potential targets for anticancer treatment. Drug Resist. Updat. 9, 134–141 (2006).
Poppe, D. et al. Azathioprine suppresses ezrin-radixin-moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J. Immunol. 176, 640–651 (2006).
Peterson, Y. K., Kelly, P., Weinbaum, C. A. & Casey, P. J. A novel protein geranylgeranyltransferase-I inhibitor with high potency, selectivity, and cellular activity. J. Biol. Chem. 281, 12445–12450 (2006). This paper highlights the development of GGTase I inhibitors (GGTIs) that demonstrate selectivity for monomeric versus heterotrimeric G proteins (such as GGTI DU40).
Lewis, K. D. et al. A Phase II open-label trial of apomine (SR-45023A) in patients with refractory melanoma. Invest. New Drugs 24, 89–94 (2006).
Kim, W. S. et al. Phase II study of high-dose lovastatin in patients with advanced gastric adenocarcinoma. Invest. New Drugs 19, 81–83 (2001).
Knoxx, J. J. et al. A Phase I trial of prolonged administration of lovastatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or of the cervix. Eur. J. Cancer 41, 523–530 (2005).
van der Spek, E. et al. Dose-finding study of high-dose simvastatin combined with standard chemotherapy in patients with relapsed or refractory myeloma or lymphoma. Haematologica 91, 542–545 (2006).
Blumenschein, G. et al. O-082. A randomized Phase III trial comparing lonafarnib/carboplatin/paclitaxel versus carboplatin/paclitaxel (CP) in chemotherapy-naive patients with advanced or metastatic non-small cell lung cancer (NSCLC). Lung Cancer 49 (Suppl. 2), 30 (2005).
Acknowledgements
The authors would like to thank A. Konstantinopoulou for her invaluable help with the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Rhabdomyolysis
-
The breakdown of muscle fibres, resulting in the release of muscle-fibre contents into the circulation. Some of these are toxic to the kidney.
- Osteoclast
-
Bone cell that has a key role in bone resorption.
- QT prolongation
-
The QT interval represents the time for electrical activation and inactivation of the ventricles, the pumping chambers of the heart. Prolongation of the QT interval can result in potentially lethal arrhythmias (some of which are known as torsades de pointes).
- Paraesthesia
-
A sensation of tingling, pricking or numbness of the skin with no apparent physical cause.
- Median effect isobologram analysis
-
A method for evaluating drug interactions such as synergism, additive effects or antagonism.
Rights and permissions
About this article
Cite this article
Konstantinopoulos, P., Karamouzis, M. & Papavassiliou, A. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov 6, 541–555 (2007). https://doi.org/10.1038/nrd2221
Issue Date:
DOI: https://doi.org/10.1038/nrd2221
This article is cited by
-
Oncogenic KRAS mutation confers chemoresistance by upregulating SIRT1 in non-small cell lung cancer
Experimental & Molecular Medicine (2023)
-
The effect of inhibition of receptor tyrosine kinase AXL on DNA damage response in ovarian cancer
Communications Biology (2023)
-
An antioxidant ameliorates allergic airway inflammation by inhibiting HDAC 1 via HIF-1α/VEGF axis suppression in mice
Scientific Reports (2023)
-
Statins and the risk of gastric, colorectal, and esophageal cancer incidence and mortality: a cohort study based on data from the Korean national health insurance claims database
Journal of Cancer Research and Clinical Oncology (2022)
-
Statins: a repurposed drug to fight cancer
Journal of Experimental & Clinical Cancer Research (2021)