Abstract
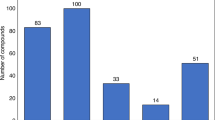
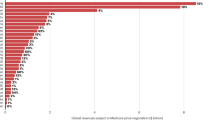
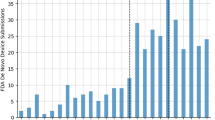
For nearly 15 years after the passage of the Hatch–Waxman Act in 1984, generics drug companies took little advantage of its provisions, which provided financial incentives to them for challenging the patents of branded pharmaceutical products. However, during the past 3–5 years, generics manufacturers have dramatically increased the number of patent challenges. Although these challenges can certainly benefit consumers and payers, the number of challenges puts many innovator companies at risk, which they argue is detrimental to future R&D spending. If many of the challenges are successful, then the increase in challenges could in turn be detrimental to generics, and the system itself might therefore be due for a re-balance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Beers, D. O. Generic and Innovator Drugs: A Guide to FDA Approval Requirements 5th Edn (Aspen Law, New York, 1999).
United States Federal Trade Commission. Generic Drug Entry Prior to Patent Expiration: An FTC Study (Federal Trade Commission; 2002).
US Food and Drug Administration, Office of Generic Drugs. Paragraph IV Patent Certifications [online], <http://www.fda.gov/cder/ogd/ppiv.htm> (2004).
ParagraphFour.com. The Paragraph Four Report [online], <http://www.paragraphfour.com> (2004).
Medicare Modernization Act. Medicare Prescription Drug, Improvement, and Modernization Act of 2003, Public Law 108–173, December 8 (2003).
DiMasi, J. A., Hansen, R. W. & Grabowski, H. G. The price of innovation: new estimates of drug development costs. J. Health Econ. 22, 151–185 (2003).
IMS Health. IMS reports 11.5 percent dollar growth in '03 U.S. prescription sales. Press Release [online], (2004).
Acknowledgements
The author would like to acknowledge F. Cohen and D. Snow for their contributions to this article.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
G.G. is an editor of the Paragraph Four Report, and is a shareholder in Pfizer and Teva Pharmaceuticals.
Related links
Rights and permissions
About this article
Cite this article
Glass, G. Pharmaceutical patent challenges — time for reassessment?. Nat Rev Drug Discov 3, 1057–1062 (2004). https://doi.org/10.1038/nrd1581
Issue Date:
DOI: https://doi.org/10.1038/nrd1581
This article is cited by
-
Patent cliff and strategic switch: exploring strategic design possibilities in the pharmaceutical industry
SpringerPlus (2016)
-
Patent watch
Nature Reviews Drug Discovery (2008)
-
Growth of the Asian health-care market: global implications for the pharmaceutical industry
Nature Reviews Drug Discovery (2007)