Key Points
-
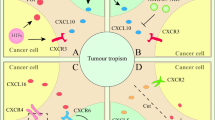
Mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells, actively contribute to the formation of the tumour microenvironment, wherein they are converted into tumour-associated MSCs (TA-MSCs).
-
TA-MSCs regulate tumour growth, metastasis and responses to chemotherapy and radiotherapy by producing growth factors, chemokines and cytokines.
-
TA-MSCs have an important role in regulating tumour immunity. Blocking TA-MSC-associated immunosuppressive factors could unleash antitumour immune responses.
-
Novel antitumour therapeutic strategies can be developed by targeting TA-MSC-produced factors that promote tumour growth, metastasis and drug resistance.
-
Owing to their tumour-tropic nature, in vitro-expanded MSCs can be genetically engineered to express antitumour agents at the tumour site to specifically kill tumour cells or to enhance antitumour immune responses.
Abstract
Mesenchymal stem cells, also known as mesenchymal stromal cells (MSCs), exist in many tissues and are known to actively migrate to sites of tissue injury, where they participate in wound repair. Tumours can be considered “wounds that never heal” and, in response to cues from a tumour, MSCs are continuously recruited to and become integral components of the tumour microenvironment. Recently, it has become apparent that such tumour-associated MSCs (TA-MSCs) have an active role in tumour initiation, promotion, progression and metastasis. In this Review, we discuss recent advances in our understanding of the pathogenic role of TA-MSCs in regulating the survival, proliferation, migration and drug resistance of tumour cells, as well as the influence of MSCs on the immune status of the tumour microenvironment. Moreover, we discuss therapeutic approaches that target TA-MSC upstream or downstream modulators or use MSCs as vehicles for the delivery of tumoricidal agents. It is anticipated that new insights into the functions of TA-MSCs will lead to the development of novel therapeutic strategies against tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Friedenstein, A. J., Chailakhjan, R. K. & Lalykina, K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 3, 393–403 (1970).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 (2008).
Shi, Y. et al. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 33, 136–143 (2012).
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014).
Quante, M. et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19, 257–272 (2011). This publication demonstrates that CAFs originate from BM-MSCs in a TGF-β and SDF1-dependent manner, and that CAFs have a critical role in creating a niche that promotes tumour progression.
Le Blanc, K. et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441 (2004).
Zhang, Z. et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 27 (Suppl. 2), 112–120 (2012).
Le Blanc, K. et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371, 1579–1586 (2008).
Sun, L. et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 62, 2467–2475 (2010).
Vojtassak, J. et al. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol. Lett. 27 (Suppl. 2), 134–137 (2006).
Caplan, A. I. & Correa, D. The MSC: an injury drugstore. Cell Stem Cell 9, 11–15 (2011).
Wang, Y., Chen, X., Cao, W. & Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 15, 1009–1016 (2014). This paper raises the concept of the plasticity of MSCs in immunomodulation. Depending on the inflammatory environment, MSCs can either inhibit or enhance immune responses in pathological conditions.
Ren, G. et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell 11, 812–824 (2012). This investigation delineates the characteristics of tumour-associated MSCs and their tumour-promoting effects through the recruitment of macrophages.
Shah, K. Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 64, 739–748 (2012).
Hu, Y. L., Fu, Y. H., Tabata, Y. & Gao, J. Q. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J. Control. Release 147, 154–162 (2010).
Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 8, 98–101 (1989).
Dvorak, H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (1986).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Roodhart, J. M. et al. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell 20, 370–383 (2011). This investigation reveals that MSCs can provide chemoresistance in tumour-bearing mice receiving platinum-analog-based cancer treatment through the production of PUFAs.
Kidd, S. et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 27, 2614–2623 (2009). By using firefly luciferase-labelled MSCs and different models of wounds and tumours, this study elegantly demonstrates a specific tropism of MSCs for tumour and wound sites.
Nakamizo, A. et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 65, 3307–3318 (2005).
Starzynska, T. et al. An intensified systemic trafficking of bone marrow-derived stem/progenitor cells in patients with pancreatic cancer. J. Cell. Mol. Med. 17, 792–799 (2013).
Alm, J. J. et al. Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. J. Orthop. Res. 28, 1634–1642 (2010).
Hoogduijn, M. J. et al. No evidence for circulating mesenchymal stem cells in patients with organ injury. Stem Cells Dev. 23, 2328–2335 (2014).
Zvaifler, N. J. et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2, 477–488 (2000).
Stagg, J. Mesenchymal stem cells in cancer. Stem Cell Rev. 4, 119–124 (2008).
Jung, Y. et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 4, 1795 (2013).
Dwyer, R. M. et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin. Cancer Res. 13, 5020–5027 (2007).
Gao, H., Priebe, W., Glod, J. & Banerjee, D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells 27, 857–865 (2009).
Spaeth, E., Klopp, A., Dembinski, J., Andreeff, M. & Marini, F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 15, 730–738 (2008).
Wagner, W. et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 33, 1402–1416 (2005).
Waite, K. A. & Eng, C. From developmental disorder to heritable cancer: it's all in the BMP/TGF-β family. Nat. Rev. Genet. 4, 763–773 (2003).
McLean, K. et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J. Clin. Invest. 121, 3206–3219 (2011).
Roccaro, A. M. et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Invest. 123, 1542–1555 (2013).
Ren, G. et al. Tumor resident mesenchymal stromal cells endow naive stromal cells with tumor-promoting properties. Oncogene 33, 4016–4020 (2014).
Lin, L. Y. et al. Tumour cell-derived exosomes endow mesenchymal stromal cells with tumour-promotion capabilities. Oncogene http://dx.doi.org/10.1038/onc.2016.131 (2016).
Mishra, P. J. et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 68, 4331–4339 (2008).
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006).
Madar, S., Goldstein, I. & Rotter, V. 'Cancer associated fibroblasts'—more than meets the eye. Trends Mol. Med. 19, 447–453 (2013).
Worthley, D. L. et al. Human gastrointestinal neoplasia-associated myofibroblasts can develop from bone marrow-derived cells following allogeneic stem cell transplantation. Stem Cells 27, 1463–1468 (2009).
Ishii, G. et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem. Biophys. Res. Commun. 309, 232–240 (2003).
Direkze, N. C. et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 64, 8492–8495 (2004).
Shinagawa, K. et al. Stroma-directed imatinib therapy impairs the tumor-promoting effect of bone marrow-derived mesenchymal stem cells in an orthotopic transplantation model of colon cancer. Int. J. Cancer 132, 813–823 (2013).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012).
Dulauroy, S., Di Carlo, S. E., Langa, F., Eberl, G. & Peduto, L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 18, 1262–1270 (2012).
Driskell, R. R. et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281 (2013).
Klemm, F. & Joyce, J. A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 25, 198–213 (2015).
Koh, B. I. & Kang, Y. The pro-metastatic role of bone marrow-derived cells: a focus on MSCs and regulatory T cells. EMBO Rep. 13, 412–422 (2012).
McIntosh, K. R., Mosca, J. D. & Klyushnenkova, E. N. Mesenchymal stem cells for prevention and treatment of immune responses in transplantation. WO Patent 1999047163 A2 (1998).
Ren, G. et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 (2008). This publication formally proves that MSCs are benign and their immunosuppressive property is licensed by inflammatory cytokine combinations.
Patel, S. A. et al. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-β. J. Immunol. 184, 5885–5894 (2010).
Li, W. et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 19, 1505–1513 (2012).
Ling, W. et al. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 74, 1576–1587 (2014).
Ren, G. et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 27, 1954–1962 (2009).
Zamarron, B. F. & Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 7, 651–658 (2011).
Zaidi, M. R. & Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 17, 6118–6124 (2011).
Han, X. et al. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 21, 1758–1768 (2014).
Xu, C. et al. TGF-β promotes immune responses in the presence of mesenchymal stem cells. J. Immunol. 192, 103–109 (2014).
David, C. J. et al. TGF-β tumor suppression through a lethal EMT. Cell 164, 1015–1030 (2016).
Djouad, F. et al. Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation 82, 1060–1066 (2006).
Montesinos, J. J. et al. In vitro evidence of the presence of mesenchymal stromal cells in cervical cancer and their role in protecting cancer cells from cytotoxic T cell activity. Stem Cells Dev. 22, 2508–2519 (2013).
Lin, R. et al. Bone marrow-derived mesenchymal stem cells favor the immunosuppressive T cells skewing in a Helicobacter pylori model of gastric cancer. Stem Cells Dev. 22, 2836–2848 (2013).
Hsu, W. T. et al. Prostaglandin E2 potentiates mesenchymal stem cell-induced IL-10+IFN-γ+CD4+ regulatory T cells to control transplant arteriosclerosis. J. Immunol. 190, 2372–2380 (2013).
Bernardo, M. E. & Fibbe, W. E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13, 392–402 (2013).
Roncarolo, M. G. et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 212, 28–50 (2006).
Vignali, D. A., Collison, L. W. & Workman, C. J. How regulatory T cells work. Nat. Rev. Immunol. 8, 523–532 (2008).
Roberts, E. W. et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 210, 1137–1151 (2013).
Liao, D., Luo, Y., Markowitz, D., Xiang, R. & Reisfeld, R. A. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS ONE 4, e7965 (2009).
Shen, K. et al. Suicide gene-engineered stromal cells reveal a dynamic regulation of cancer metastasis. Sci. Rep. 6, 21239 (2016).
Narra, K. et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting fibroblast activation protein in patients with metastatic colorectal cancer. Cancer Biol. Ther. 6, 1691–1699 (2007).
Hofheinz, R. D. et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 26, 44–48 (2003).
Ozdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Santos, A. M., Jung, J., Aziz, N., Kissil, J. L. & Pure, E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Invest. 119, 3613–3625 (2009).
Francois, M. et al. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood 114, 2632–2638 (2009).
Chan, J. L. et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-γ. Blood 107, 4817–4824 (2006).
Andreu, P. et al. FcRγ activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 17, 121–134 (2010).
Ammirante, M., Luo, J. L., Grivennikov, S., Nedospasov, S. & Karin, M. B-Cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464, 302–305 (2010).
Zhang, Y., Gallastegui, N. & Rosenblatt, J. D. Regulatory B cells in anti-tumor immunity. Int. Immunol. 27, 521–530 (2015).
Kobayashi, T. et al. B cells promote tumor immunity against B16F10 melanoma. Am. J. Pathol. 184, 3120–3129 (2014).
Corcione, A. et al. Human mesenchymal stem cells modulate B-cell functions. Blood 107, 367–372 (2006).
Asari, S. et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp. Hematol. 37, 604–615 (2009).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Qian, B. et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE 4, e6562 (2009).
Yoshikawa, K. et al. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci. 103, 2012–2020 (2012).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Wculek, S. K. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015).
Abumaree, M. H. et al. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev. 9, 620–641 (2013).
Nemeth, K. et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 15, 42–49 (2009).
Hof-Nahor, I. et al. Human mesenchymal stem cells shift CD8+ T cells towards a suppressive phenotype by inducing tolerogenic monocytes. J. Cell Sci. 125, 4640–4650 (2012).
Akiyama, K. et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 10, 544–555 (2012).
Bonecchi, R. et al. Chemokines and chemokine receptors: an overview. Front. Biosci. 14, 540–551 (2009).
Guilloton, F. et al. Mesenchymal stromal cells orchestrate follicular lymphoma cell niche through the CCL2-dependent recruitment and polarization of monocytes. Blood 119, 2556–2567 (2012).
Yu, P. F. et al. TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene http://dx.doi.org/10.1038/onc.2016.217 (2016).
Chaturvedi, P., Gilkes, D. M., Takano, N. & Semenza, G. L. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc. Natl Acad. Sci. USA 111, E2120–E2129 (2014).
Zhang, L. et al. Bone marrow mesenchymal stem cells suppress metastatic tumor development in mouse by modulating immune system. Stem Cell Res. Ther. 6, 45 (2015).
Beckermann, B. M. et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br. J. Cancer 99, 622–631 (2008).
De Boeck, A. et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 62, 550–560 (2013).
Crovello, C. S., Lai, C., Cantley, L. C. & Carraway, K. L. III. Differential signaling by the epidermal growth factor-like growth factors neuregulin-1 and neuregulin-2. J. Biol. Chem. 273, 26954–26961 (1998).
Mandel, K. et al. Mesenchymal stem cells directly interact with breast cancer cells and promote tumor cell growth in vitro and in vivo. Stem Cells Dev. 22, 3114–3127 (2013).
Rosova, I., Dao, M., Capoccia, B., Link, D. & Nolta, J. A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26, 2173–2182 (2008).
Wei, L., Fraser, J. L., Lu, Z. Y., Hu, X. & Yu, S. P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol. Dis. 46, 635–645 (2012).
Chen, J. et al. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 74, 879–889 (2008).
Huang, W. H. et al. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 32, 4343–4354 (2013).
Sun, B. et al. Correlation between melanoma angiogenesis and the mesenchymal stem cells and endothelial progenitor cells derived from bone marrow. Stem Cells Dev. 14, 292–298 (2005).
Al-Khaldi, A. et al. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 10, 621–629 (2003).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005).
Petit, I., Jin, D. & Rafii, S. The SDF-1–CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 28, 299–307 (2007).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
Bexell, D. et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol. Ther. 17, 183–190 (2009).
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Gupta, G. P. & Massague, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 4, 540–550 (2004).
Zlotnik, A., Burkhardt, A. M. & Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 11, 597–606 (2011).
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
Zhang, X. H. et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154, 1060–1073 (2013). This study demonstrates that CXCL12 and IGF1 produced by CAFs have crucial roles in selecting the seeds in the primary tumour for metastasis to CXCL12-riched organs.
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563 (2007). This investigation demonstrated that MSCs educated by cancer cells secrete the chemokine CCL5, which then acts on cancer cells to promote the motility, invasion and metastasis of cancer cells via CCR5 signalling.
Mi, Z. et al. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis 32, 477–487 (2011).
Sullivan, R. & Graham, C. H. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 26, 319–331 (2007).
Chan, D. A. & Giaccia, A. J. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 26, 333–339 (2007).
Dayan, F., Mazure, N. M., Brahimi-Horn, M. C. & Pouyssegur, J. A dialogue between the hypoxia-inducible factor and the tumor microenvironment. Cancer Microenviron. 1, 53–68 (2008).
Chaturvedi, P. et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J. Clin. Invest. 123, 189–205 (2013).
Kalluri, R. EMT: when epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 119, 1417–1419 (2009).
Luo, J. et al. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene 33, 2768–2778 (2014).
Luga, V. et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151, 1542–1556 (2012).
Chen, R. et al. Regulation of IKKβ by miR-199a affects NF-κB activity in ovarian cancer cells. Oncogene 27, 4712–4723 (2008).
Cuiffo, B. G. et al. MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell 15, 762–774 (2014).
Enard, W. et al. Molecular evolution of FOXP2, a gene involved in speech and language. Nature 418, 869–872 (2002).
Gilkes, D. M., Semenza, G. L. & Wirtz, D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer 14, 430–439 (2014).
Calvo, F. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15, 637–646 (2013).
Ries, C. et al. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 109, 4055–4063 (2007).
Deryugina, E. I. & Quigley, J. P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 25, 9–34 (2006).
El-Haibi, C. P. et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc. Natl Acad. Sci. USA 109, 17460–17465 (2012).
Gilkes, D. M., Bajpai, S., Chaturvedi, P., Wirtz, D. & Semenza, G. L. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 288, 10819–10829 (2013).
Mao, Y., Keller, E. T., Garfield, D. H., Shen, K. & Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 32, 303–315 (2013).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Psaila, B. & Lyden, D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer 9, 285–293 (2009).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Hiratsuka, S. et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2, 289–300 (2002).
Li, X. et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl Cancer Inst. 100, 672–679 (2008).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Visvader, J. E. Cells of origin in cancer. Nature 469, 314–322 (2011).
Bissell, M. J. & Labarge, M. A. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell 7, 17–23 (2005).
Huijbers, A. et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann. Oncol. 24, 179–185 (2013).
Cukierman, E. & Bassi, D. E. The mesenchymal tumor microenvironment: a drug-resistant niche. Cell Adh. Migr. 6, 285–296 (2012).
McMillin, D. W., Negri, J. M. & Mitsiades, C. S. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat. Rev. Drug Discov. 12, 217–228 (2013).
Muerkoster, S. et al. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1β. Cancer Res. 64, 1331–1337 (2004).
Lotti, F. et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J. Exp. Med. 210, 2851–2872 (2013).
Scherzed, A. et al. BMSC enhance the survival of paclitaxel treated squamous cell carcinoma cells in vitro. Cancer Biol. Ther. 11, 349–357 (2011).
Lis, R. et al. Tumor associated mesenchymal stem cells protects ovarian cancer cells from hyperthermia through CXCL12. Int. J. Cancer 128, 715–725 (2011).
Hellevik, T. et al. Changes in the secretory profile of NSCLC-associated fibroblasts after ablative radiotherapy: potential impact on angiogenesis and tumor growth. Transl. Oncol. 6, 66–74 (2013).
Barcellos-Hoff, M. H. & Ravani, S. A. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 60, 1254–1260 (2000).
Saigusa, S. et al. Cancer-associated fibroblasts correlate with poor prognosis in rectal cancer after chemoradiotherapy. Int. J. Oncol. 38, 655–663 (2011).
Isella, C. et al. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 47, 312–319 (2015).
Wilson, T. R. et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 487, 505–509 (2012).
Straussman, R. et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487, 500–504 (2012). References 156 and 157 unravel a key role of HGF produced by CAFs in conferring chemoresistance in BRAF-mutant melanoma cells.
Crawford, Y. et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15, 21–34 (2009).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Ono, M. et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 7, ra63 (2014).
Boelens, M. C. et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 159, 499–513 (2014).
Munoz, J. L. et al. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol. Ther. Nucleic Acids 2, e126 (2013).
US National Library of Medicine. Clinicaltrials.govhttps://www.clinicaltrials.gov/ct2/show/NCT01983709?term=NCT01983709&rank=1 (2016).
US National Library of Medicine. Clinicaltrials.govhttps://www.clinicaltrials.gov/ct2/show/NCT02068794?term=NCT02068794&rank=1 (2016).
US National Library of Medicine. Clinicaltrials.govhttps://www.clinicaltrials.gov/ct2/show/NCT02530047?term=NCT02530047&rank=1 (2016).
US National Library of Medicine. Clinicaltrials.govhttps://www.clinicaltrials.gov/ct2/show/NCT02008539?term=NCT02008539&rank=1 (2015).
Zhang, Y. et al. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat. Cell Biol. 15, 284–294 (2013).
Ho, I. A. et al. Matrix metalloproteinase-1-mediated mesenchymal stem cell tumor tropism is dependent on crosstalk with stromal derived growth factor 1/C-X-C chemokine receptor 4 axis. FASEB J. 28, 4359–4368 (2014).
Luz-Crawford, P. et al. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS ONE 7, e45272 (2012).
Su, J. et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 21, 388–396 (2014).
Nayak, A. et al. A Phase I study of NLG919 for adult patients with recurrent advanced solid tumors. J. Immunother. Cancer 2, (Suppl. 3), P250 (2014).
Muller, A. J., DuHadaway, J. B., Donover, P. S., Sutanto-Ward, E. & Prendergast, G. C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 11, 312–319 (2005).
Holmgaard, R. B., Zamarin, D., Munn, D. H., Wolchok, J. D. & Allison, J. P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 210, 1389–1402 (2013).
Lob, S., Konigsrainer, A., Rammensee, H. G., Opelz, G. & Terness, P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat. Rev. Cancer 9, 445–452 (2009). This comprehensive review summarizes recent advances in the development and use of IDO inhibitors for cancer treatment.
Soliman, H. H. et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget 5, 8136–8146 (2014).
Novitskiy, S. V. & Moses, H. L. Turn off the IDO: will clinical trials be successful? Cancer Discov. 2, 673–675 (2012).
Xu, C. et al. Interferon-α-secreting mesenchymal stem cells exert potent antitumor effect in vivo. Oncogene 33, 5047–5052 (2014).
Niess, H. et al. Treatment of advanced gastrointestinal tumors with genetically modified autologous mesenchymal stromal cells (TREAT-ME1): study protocol of a phase I/II clinical trial. BMC Cancer 15, 237 (2015).
Mirzaei, H. et al. Application of mesenchymal stem cells in melanoma: a potential therapeutic strategy for delivery of targeted agents. Curr. Med. Chem. 23, 455–463 (2016).
Dwyer, R. M., Khan, S., Barry, F. P., O'Brien, T. & Kerin, M. J. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res. Ther. 1, 25 (2010).
Kucerova, L., Altanerova, V., Matuskova, M., Tyciakova, S. & Altaner, C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 67, 6304–6313 (2007).
Kucerova, L. et al. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J. Gene Med. 10, 1071–1082 (2008).
Fischer, U. M. et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 18, 683–692 (2009).
Cavarretta, I. T. et al. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol. Ther. 18, 223–231 (2010).
Yong, R. L. et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Δ24-RGD to human gliomas. Cancer Res. 69, 8932–8940 (2009).
Kanehira, M. et al. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 14, 894–903 (2007).
Pan, G. et al. The receptor for the cytotoxic ligand TRAIL. Science 276, 111–113 (1997).
Loebinger, M. R., Eddaoudi, A., Davies, D. & Janes, S. M. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 69, 4134–4142 (2009).
Grisendi, G. et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 70, 3718–3729 (2010).
Lee, R. H., Yoon, N., Reneau, J. C. & Prockop, D. J. Preactivation of human MSCs with TNF-α enhances tumor-suppressive activity. Cell Stem Cell 11, 825–835 (2012). This study shows that TNF-treated MSCs express TRAIL and DKK3, and exhibit enhanced tumour-suppressive effects.
Xiang, H., Nguyen, C. B., Kelley, S. K., Dybdal, N. & Escandon, E. Tissue distribution, stability, and pharmacokinetics of Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand in human colon carcinoma COLO205 tumor-bearing nude mice. Drug Metab. Dispos. 32, 1230–1238 (2004).
Kelley, S. K. et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J. Pharmacol. Exp. Ther. 299, 31–38 (2001).
Dunn, G. P., Koebel, C. M. & Schreiber, R. D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848 (2006).
Studeny, M. et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 62, 3603–3608 (2002).
Yang, X., Du, J., Xu, X., Xu, C. & Song, W. IFN-γ-secreting-mesenchymal stem cells exert an antitumor effect in vivo via the TRAIL pathway. J. Immunol. Res. 2014, 318098 (2014).
Elzaouk, L., Moelling, K. & Pavlovic, J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp. Dermatol. 15, 865–874 (2006).
Xin, H. et al. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells 25, 1618–1626 (2007).
Xin, H. et al. Intratracheal delivery of CX3CL1-expressing mesenchymal stem cells to multiple lung tumors. Mol. Med. 15, 321–327 (2009).
Nelson, D., Fisher, S. & Robinson, B. The “Trojan Horse” approach to tumor immunotherapy: targeting the tumor microenvironment. J. Immunol. Res. 2014, 789069 (2014).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 (2006).
Lv, F. J., Tuan, R. S., Cheung, K. M. & Leung, V. Y. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32, 1408–1419 (2014).
Sacchetti, B. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007).
Serafini, M. et al. Establishment of bone marrow and hematopoietic niches in vivo by reversion of chondrocyte differentiation of human bone marrow stromal cells. Stem Cell Res. 12, 659–672 (2014).
Mendez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010). The experiments in this publication show that nestin is a marker for tracing bone marrow MSCs and the partnership between nestin+ cells and hematopoietic stem cells forms a unique bone marrow niche.
Hess, R., Pino, A. M., Rios, S., Fernandez, M. & Rodriguez, J. P. High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. J. Cell Biochem. 94, 50–57 (2005).
Le Blanc, K. & Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 12, 383–396 (2012).
Ohlund, D., Elyada, E. & Tuveson, D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 211, 1503–1523 (2014).
Moinfar, F. et al. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 60, 2562–2566 (2000).
Menendez, P. et al. Bone marrow mesenchymal stem cells from infants with MLL-AF4+ acute leukemia harbor and express the MLL-AF4 fusion gene. J. Exp. Med. 206, 3131–3141 (2009).
Kurose, K. et al. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat. Genet. 32, 355–357 (2002).
Weber, F. et al. Microenvironmental genomic alterations and clinicopathological behavior in head and neck squamous cell carcinoma. JAMA 297, 187–195 (2007).
Hill, R., Song, Y., Cardiff, R. D. & Van Dyke, T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell 123, 1001–1011 (2005). This study uses a mouse prostate cancer model and finds that oncogenic stress imposes selective pressure on the mesenchyme with loss of p53.
Huang, Y. et al. p53 regulates mesenchymal stem cell-mediated tumor suppression in a tumor microenvironment through immune modulation. Oncogene 33, 3830–3838 (2014).
Patocs, A. et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N. Engl. J. Med. 357, 2543–2551 (2007).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006).
Condamine, T., Ramachandran, I., Youn, J. I. & Gabrilovich, D. I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 66, 97–110 (2015).
Yan, C. et al. Human umbilical cord mesenchymal stem cells as vehicles of CD20-specific TRAIL fusion protein delivery: a double-target therapy against non-Hodgkin's lymphoma. Mol. Pharm. 10, 142–151 (2013).
Acknowledgements
We thank A. I. Roberts for critically reviewing and discussing the manuscript. We are indebted to those whose work is not discussed owing to space limitations. This work is supported by National Natural Science Foundation of China (81530043, 81330046), the Ministry of Science and Technology of China (2015CB964500), the Scientific Innovation Project of the Chinese Academy of Science (XDA01040100), the Suzhou Science and Technology Program (SZS201616), the Shanghai Rising-Star Program (14QA1404200), the Youth Innovation Promotion Association research fund from the Chinese Acadmey of Sciences (2060206),and the Department of Science and Technology of Jiangsu Province research fund (BE2016671).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Inducible nitric oxide synthase
-
(iNOS). One of the key enzymes generating nitric oxide from L-arginine. The expression of iNOS is usually regulated by inflammatory cytokines.
- Indoleamine 2,3-dioxygenase
-
(IDO). A rate-limiting enzyme that catalyses L-tryptophan catabolism through the kynurenine pathway.
- ARE/poly(U)-binding/degradation factor 1
-
(AUF1). A regulatory protein that controls mRNA stability through its interaction with the adenosine- and uridine-rich regions of the target mRNA.
- Secretome
-
All of the factors secreted by cells, tissues, organs and organisms in a given biological setting.
- 5-Fluorouracil
-
(5-FU). A drug used for the treatment of tumours. 5-FU can inhibit the synthesis of thymidylate and thus induce the cell death of tumour cells.
- 1-Methyl-DL-tryptophan
-
(1-MT). A competitive inhibitor of indoleamine 2, 3-dioxygenase.
- Cytosine deaminase
-
(CD). A hydrolase acting on carbon–nitrogen bonds other than peptide bonds, specifically in cyclic amidines. CD converts non-toxic prodrug 5-fluorocytosine (5-FC) to the tumour-toxic chemotherapeutic agent 5-fluorouracil (5-FU).
Rights and permissions
About this article
Cite this article
Shi, Y., Du, L., Lin, L. et al. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov 16, 35–52 (2017). https://doi.org/10.1038/nrd.2016.193
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2016.193
This article is cited by
-
Tumor-derived small extracellular vesicles facilitate omental metastasis of ovarian cancer by triggering activation of mesenchymal stem cells
Cell Communication and Signaling (2024)
-
Immunosuppressive MFAP2+ cancer associated fibroblasts conferred unfavorable prognosis and therapeutic resistance in gastric cancer
Cellular Oncology (2024)
-
Shining the light on mesenchymal stem cell-derived exosomes in breast cancer
Stem Cell Research & Therapy (2023)
-
Development and validation of a glioma-associated mesenchymal stem cell-related gene prognostic index for predicting prognosis and guiding individualized therapy in glioma
Stem Cell Research & Therapy (2023)
-
Mesenchymal stromal cells promote the drug resistance of gastrointestinal stromal tumors by activating the PI3K-AKT pathway via TGF-β2
Journal of Translational Medicine (2023)