Key Points
-
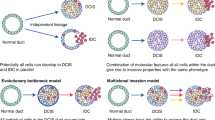
Atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), and ductal carcinoma in situ (DCIS) are high-risk breast lesions; flat epithelial atypia (FEA) has an uncertain risk level
-
ADH or ALH, and LCIS are associated with relative risk of breast cancer development, in either breast, of approximately 4 and 10, respectively
-
The degree of risk associated with ALH, ADH, and LCIS is sufficient to justify a discussion of chemoprevention in healthy women, particularly those who are premenopausal at diagnosis
-
DCIS is considered a precursor lesion and is routinely treated by surgical excision, sometimes necessitating mastectomy; radiotherapy and/or endocrine therapy are often added, although neither modality reduces breast-cancer-specific mortality
-
Clinicopathological features or molecular alterations identifying the individuals with high-risk breast lesions that will progress to invasive breast carcinoma remain to be identified
-
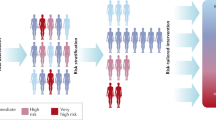
Until predictors of invasive carcinoma are found, management strategies must be defined by risk at the population level, rather than individual level, which might result in undertreatment or overtreatment in individual patients
Abstract
High-risk breast lesions, which comprise benign lesions and in situ carcinomas (lobular carcinoma in situ and ductal carcinoma in situ), are clinically, morphologically, and biologically heterogeneous and are associated with an increased risk of invasive breast cancer development, albeit to varying degrees. Recognition and proactive management of such lesions can help to prevent progression to invasive disease, and might, therefore, reduce breast cancer incidence, morbidity, and mortality. However, this opportunity comes with the possibility of overdiagnosis and overtreatment, necessitating risk-based intervention. Notably, despite the progress in defining the molecular changes associated with carcinogenesis, alterations identifying the individuals with high-risk lesions that will progress to invasive carcinoma remain to be identified. Thus, until reproducible clinicopathological or molecular features predicting an individual's risk of breast cancer are found, management strategies must be defined by population-level risks as determined by models such as the Gail or IBIS models, as well as patient attitudes toward the risks and benefits of interventions. Herein, we review the contemporary approaches to diagnosis and management of high-risk breast lesions. Progress in this area will ultimately be dependent on the ability to individualize risk prediction through better definition of the key drivers in the carcinogenic process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dupont, W. D. & Page, D. L. Risk factors for breast cancer in women with proliferative breast disease. N. Engl. J. Med. 312, 146–151 (1985).
Fitzgibbons, P. L., Henson, D. E. & Hutter, R. V. Benign breast changes and the risk for subsequent breast cancer: an update of the 1985 consensus statement. Cancer Committee of the College of American Pathologists. Arch. Pathol. Lab. Med. 122, 1053–1055 (1998).
Collins, L. C. et al. The influence of family history on breast cancer risk in women with biopsy-confirmed benign breast disease: results from the Nurses' Health Study. Cancer 107, 1240–1247 (2006).
Collins, L. C. et al. Magnitude and laterality of breast cancer risk according to histologic type of atypical hyperplasia: results from the Nurses' Health Study. Cancer 109, 180–187 (2007).
Hartmann, L. C. et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. Cancer Prev. Res. (Phila.) 7, 211–217 (2014).
Rubin, E., Visscher, D. W., Alexander, R. W., Urist, M. M. & Maddox, W. A. Proliferative disease and atypia in biopsies performed for nonpalpable lesions detected mammographically. Cancer 61, 2077–2082 (1988).
Lakhani, S., Ellis, I. O., Schnitt, S. J., Tan, P. H. & van de Vijver, M. J. (eds) WHO Classification of Tumours of the Breast 4th edn 77–89 (IARC Press, 2012).
Degnim, A. C. et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J. Clin. Oncol. 25, 2671–2677 (2007).
Page, D. L., Dupont, W. D., Rogers, L. W. & Rados, M. S. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 55, 2698–2708 (1985).
Page, D. L. et al. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet 361, 125–129 (2003).
Gail, M. H. et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J. Natl Cancer Inst. 81, 1879–1886 (1989).
Costantino, J. P. et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J. Natl Cancer Inst. 91, 1541–1548 (1999).
Rockhill, B., Spiegelman, D., Byrne, C., Hunter, D. J. & Colditz, G. A. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J. Natl Cancer Inst. 93, 358–366 (2001).
Boughey, J. C. et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J. Clin. Oncol. 28, 3591–3596 (2010).
Allred, D. C. Molecular biomarkers of risk in premalignancy and breast cancer prevention. Cancer Prev. Res. (Phila.) 4, 1947–1952 (2011).
Tavassoli, F. A. et al. in World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Breast and Female Genital Organs (eds Tavassoli, F. A. & Devillee, P.) 65–66 (IARC Press, 2003).
Chivukula, M., Bhargava, R., Tseng, G. & Dabbs, D. J. Clinicopathologic implications of “flat epithelial atypia” in core needle biopsy specimens of the breast. Am. J. Clin. Pathol. 131, 802–808 (2009).
Khoumais, N. A. et al. Incidence of breast cancer in patients with pure flat epithelial atypia diagnosed at core-needle biopsy of the breast. Ann. Surg. Oncol. 20, 133–138 (2013).
Uzoaru, I. et al. Flat epithelial atypia with and without atypical ductal hyperplasia: to re-excise or not. Results of a 5-year prospective study. Virchows Arch. 461, 419–423 (2012).
Said, S. M. et al. Risk of developing invasive breast cancer in patients with flat epithelial atypia (FEA) in benign breast biopsies. Mod. Pathol. 27, 79A (2014).
Foote, F. W. & Stewart, F. W. Lobular carcinoma in situ: a rare form of mammary cancer. Am. J. Pathol. 17, 491–496 (1941).
Haagensen, C. D., Lane, N., Lattes, R. & Bodian, C. Lobular neoplasia (so-called lobular carcinoma in situ) of the breast. Cancer 42, 737–769 (1978).
Rosen, P. P., Kosloff, C., Lieberman, P. H., Adair, F. & Braun, D. W. Jr. Lobular carcinoma in situ of the breast. Detailed analysis of 99 patients with average follow-up of 24 years. Am. J. Surg. Pathol. 2, 225–251 (1978).
Galimberti, V., Monti, S. & Mastropasqua, M. G. DCIS and LCIS are confusing and outdated terms. They should be abandoned in favor of ductal intraepithelial neoplasia (DIN) and lobular intraepithelial neoplasia (LIN). Breast 22, 431–435 (2013).
Aulmann, S. et al. Clonality of lobular carcinoma in situ (LCIS) and metachronous invasive breast cancer. Breast Cancer Res. Treat. 107, 331–335 (2008).
Lopez-Garcia, M. A., Geyer, F. C., Lacroix-Triki, M., Marchio, C. & Reis-Filho, J. S. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology 57, 171–192 (2010).
Khoury, T. et al. Pleomorphic lobular carcinoma in situ of the breast: clinicopathological review of 47 cases. Histopathology 64, 981–993 (2014).
Page, D. L., Kidd, T. E. Jr, Dupont, W. D., Simpson, J. F. & Rogers, L. W. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum. Pathol. 22, 1232–1239 (1991).
Urban, J. A. Bilaterality of cancer of the breast. Biopsy of the opposite breast. Cancer 20, 1867–1870 (1967).
Chuba, P. J. et al. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J. Clin. Oncol. 23, 5534–5541 (2005).
Fisher, E. R. et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: twelve-year observations concerning lobular carcinoma in situ. Cancer 100, 238–244 (2004).
King, T. A. et al. Is there a role for routine screening MRI in women with LCIS? Breast Cancer Res. Treat. 142, 445–453 (2013).
Coopey, S. B. et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res. Treat. 136, 627–633 (2012).
Salvadori, B. et al. Risk of invasive cancer in women with lobular carcinoma in situ of the breast. Eur. J. Cancer 27, 35–37 (1991).
Ottesen, G. L. et al. Lobular carcinoma in situ of the female breast. Short-term results of a prospective nationwide study. The Danish Breast Cancer Cooperative Group. Am. J. Surg. Pathol. 17, 14–21 (1993).
Ernster, V. L. & Barclay, J. Increases in ductal carcinoma in situ (DCIS) of the breast in relation to mammography: a dilemma. J. Natl Cancer Inst. Monogr. 1997, 151–156 (1997).
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 63, 11–30 (2013).
Collins, L. C. et al. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses' Health Study. Cancer 103, 1778–1784 (2005).
Eusebi, V. et al. Long-term follow-up of in situ carcinoma of the breast. Semin. Diagn. Pathol. 11, 223–235 (1994).
Page, D. L., Dupont, W. D., Rogers, L. W., Jensen, R. A. & Schuyler, P. A. Continued local recurrence of carcinoma 15–25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer 76, 1197–1200 (1995).
Rosen, P. P., Braun, D. W. Jr & Kinne, D. E. The clinical significance of pre-invasive breast carcinoma. Cancer 46, 919–925 (1980).
Hughes, L. L. et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 27, 5319–5324 (2009).
Solin, L. J. et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J. Natl Cancer Inst. 105, 701–710 (2013).
Virnig, B. A., Wang, S. Y., Shamilyan, T., Kane, R. L. & Tuttle, T. M. Ductal carcinoma in situ: risk factors and impact of screening. J. Natl Cancer Inst. Monogr. 2010, 113–116 (2010).
Choi, D. X. et al. Blurry boundaries: do epithelial borderline lesions of the breast and ductal carcinoma in situ have similar rates of subsequent invasive cancer? Ann. Surg. Oncol. 20, 1302–1310 (2013).
Vandenbussche, C. J. et al. Borderline atypical ductal hyperplasia/low-grade ductal carcinoma in situ on breast needle core biopsy should be managed conservatively. Am. J. Surg. Pathol. 37, 913–923 (2013).
Bijker, N. et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J. Clin. Oncol. 24, 3381–3387 (2006).
Fisher, B. et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J. Clin. Oncol. 16, 441–452 (1998).
Wapnir, I. L. et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J. Natl Cancer Inst. 103, 478–488 (2011).
Solin, L. J. et al. Fifteen-year results of breast-conserving surgery and definitive breast irradiation for the treatment of ductal carcinoma in situ of the breast. J. Clin. Oncol. 14, 754–763 (1996).
Vincent-Salomon, A. et al. Integrated genomic and transcriptomic analysis of ductal carcinoma in situ of the breast. Clin. Cancer Res. 14, 1956–1965 (2008).
Johnson, C. E. et al. Identification of copy number alterations associated with the progression of DCIS to invasive ductal carcinoma. Breast Cancer Res. Treat. 133, 889–898 (2012).
Park, S. Y. et al. Promoter CpG island hypermethylation during breast cancer progression. Virchows Arch. 458, 73–84 (2011).
Hu, M. et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell 13, 394–406 (2008).
Cowell, C. F. et al. Progression from ductal carcinoma in situ to invasive breast cancer: revisited. Mol. Oncol. 7, 859–869 (2013).
Degnim, A. C. & King, T. A. Surgical management of high-risk breast lesions. Surg. Clin. North Am. 93, 329–340 (2013).
Brennan, M. E. et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology 260, 119–128 (2011).
Hussain, M. & Cunnick, G. H. Management of lobular carcinoma in-situ and atypical lobular hyperplasia of the breast—a review. Eur. J. Surg. Oncol. 37, 279–289 (2011).
Middleton, L. P. et al. Most lobular carcinoma in situ and atypical lobular hyperplasia diagnosed on core needle biopsy can be managed clinically with radiologic follow-up in a multidisciplinary setting. Cancer Med. 3, 492–499 (2014).
Murray, M. P. et al. Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: outcomes of prospective excision. Cancer 119, 1073–1079 (2013).
Rendi, M. H., Dintzis, S. M., Lehman, C. D., Calhoun, K. E. & Allison, K. H. Lobular in-situ neoplasia on breast core needle biopsy: imaging indication and pathologic extent can identify which patients require excisional biopsy. Ann. Surg. Oncol. 19, 914–921 (2012).
Shah-Khan, M. G. et al. Long-term follow-up of lobular neoplasia (atypical lobular hyperplasia/lobular carcinoma in situ) diagnosed on core needle biopsy. Ann. Surg. Oncol. 19, 3131–3138 (2012).
Subhawong, A. P., Subhawong, T. K., Khouri, N., Tsangaris, T. & Nassar, H. Incidental minimal atypical lobular hyperplasia on core needle biopsy: correlation with findings on follow-up excision. Am. J. Surg. Pathol. 34, 822–828 (2010).
Noel, J. C., Buxant, F. & Engohan-Aloghe, C. Immediate surgical resection of residual microcalcifications after a diagnosis of pure flat epithelial atypia on core biopsy: a word of caution. Surg. Oncol. 19, 243–246 (2010).
Piubello, Q. et al. Flat epithelial atypia on core needle biopsy: which is the right management? Am. J. Surg. Pathol. 33, 1078–1084 (2009).
Prowler, V. L. et al. Surgical excision of pure flat epithelial atypia identified on core needle breast biopsy. Breast 23, 352–356 (2014).
Abner, A. L. et al. The relation between the presence and extent of lobular carcinoma in situ and the risk of local recurrence for patients with infiltrating carcinoma of the breast treated with conservative surgery and radiation therapy. Cancer 88, 1072–1077 (2000).
Ciocca, R. M., Li, T., Freedman, G. M. & Morrow, M. Presence of lobular carcinoma in situ does not increase local recurrence in patients treated with breast-conserving therapy. Ann. Surg. Oncol. 15, 2263–2271 (2008).
Houssami, N. et al. Accuracy of screening mammography in women with a history of lobular carcinoma in situ or atypical hyperplasia of the breast. Breast Cancer Res. Treat. 145, 765–773 (2014).
Saslow, D. et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 57, 75–89 (2007).
Berg, W. A. et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 307, 1394–1404 (2012).
National Comprehensive Cancer Network (NCCN). NCCN Guideline for Breast Cancer Screening and Diagnosis. Version 1.2014 [online], (2014).
Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365, 1687–1717 (2005).
Fisher, B. et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl Cancer Inst. 97, 1652–1662 (2005).
Cuzick, J. et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 361, 296–300 (2003).
Cuzick, J. Long-term follow-up in cancer prevention trials (it ain't over 'til it's over). Cancer Prev. Res. (Phila.) 3, 689–691 (2010).
Cuzick, J. et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. http://dx.doi.org/10.1016/S1470-2045(14)71171-4.
Vogel, V. G. et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 trial: preventing breast cancer. Cancer Prev. Res. (Phila.) 3, 696–706 (2010).
Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists' Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 9, 45–53 (2008).
Cuzick, J. et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 383, 1041–1048 (2014).
Goss, P. E. et al. Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 364, 2381–2391 (2011).
Freedman, A. N. et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J. Clin. Oncol. 29, 2327–2333 (2011).
Visvanathan, K. et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 31, 2942–2962 (2013).
Oppong, B. A. & King, T. A. Recommendations for women with lobular carcinoma in situ (LCIS). Oncology (Williston Park) 25, 1051–1056 (2011).
Geiger, A. M. et al. A population-based study of bilateral prophylactic mastectomy efficacy in women at elevated risk for breast cancer in community practices. Arch. Intern. Med. 165, 516–520 (2005).
National Comprehensive Cancer Network (NCCN). NCCN Guidelines: Ductal Carcinoma in situ. Version 2.2013 [online], (2013).
Van Zee, K. J., White, J., Morrow, M. & Harris, J. R. Ductal carcinoma in situ and microinvasive carcinoma in Diseases of the Breast 5th edn (eds Harris, J. R., Lippman, M. E., Morrow, M. & Osborne, C. K.) 337–359 (Wolters Kluwer, 2014).
Correa, C. et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J. Natl Cancer Inst. Monogr. 2010, 162–177 (2010).
Kelley, L., Silverstein, M. & Guerra, L. Analyzing the risk of recurrence after mastectomy for DCIS: a new use for the USC/Van Nuys Prognostic Index. Ann. Surg. Oncol. 18, 459–462 (2011).
Owen, D. et al. Outcomes in patients treated with mastectomy for ductal carcinoma in situ. Int. J. Radiat. Oncol. Biol. Phys. 85, e129–e134 (2013).
Wells, C. J., O'Donoghue, C., Ojeda-Fournier, H., Retallack, H. E. & Esserman, L. J. Evolving paradigm for imaging, diagnosis, and management of DCIS. J. Am. Coll. Radiol. 10, 918–923 (2013).
Katz, S. J. et al. Patient involvement in surgery treatment decisions for breast cancer. J. Clin. Oncol. 23, 5526–5533 (2005).
Allred, D. C. et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J. Clin. Oncol. 30, 1268–1273 (2012).
Barnes, N. L., Dimopoulos, N., Williams, K. E., Howe, M. & Bundred, N. J. The frequency of presentation and clinico-pathological characteristics of symptomatic versus screen detected ductal carcinoma in situ of the breast. Eur. J. Surg. Oncol. 40, 249–254 (2014).
Fisher, B. et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet 353, 1993–2000 (1999).
Cuzick, J. et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 12, 21–29 (2011).
Staley, H., McCallum, I. & Bruce, J. Postoperative tamoxifen for ductal carcinoma in situ. Cochrane Database of Systematic Reviews, Issue 10. Art. No.: CD007847 http://dx.doi.org/10.1002/14651858.CD007847.pub2 (2012).
Davies, C. et al. Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013).
Burstein, H. J. et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J. Clin. Oncol. 32, 2255–2269 (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to all stages of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Morrow, M., Schnitt, S. & Norton, L. Current management of lesions associated with an increased risk of breast cancer. Nat Rev Clin Oncol 12, 227–238 (2015). https://doi.org/10.1038/nrclinonc.2015.8
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2015.8
This article is cited by
-
High‐Risk Breast Lesions Diagnosed by Ultrasound‐Guided Vacuum‐Assisted Excision
World Journal of Surgery (2023)
-
A novel chemical inhibitor suppresses breast cancer cell growth and metastasis through inhibiting HPIP oncoprotein
Cell Death Discovery (2021)
-
Positive predictive value for malignancy of uncertain malignant potential (B3) breast lesions diagnosed on vacuum-assisted biopsy (VAB): is surgical excision still recommended?
European Radiology (2021)
-
Performance of a clinical and imaging-based multivariate model as decision support tool to help save unnecessary surgeries for high-risk breast lesions
Breast Cancer Research and Treatment (2021)
-
Breast cancer screening for women at high risk: review of current guidelines from leading specialty societies
Breast Cancer (2021)