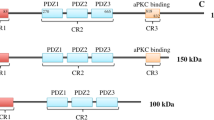
Abstract
The role of cell polarity regulators in the development of cancer has long been an enigma. Despite displaying characteristics of tumour suppressors, the core regulators of polarity are rarely mutated in tumours and there are few data from animal models to suggest that they directly contribute to cancer susceptibility, thus questioning their relevance to human carcinogenesis. However, a body of data from human tumour viruses is now providing compelling evidence of a central role for the perturbation of cell polarity in the development of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
McCaffrey, L. M. & Macara, I. G. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 21, 727–735 (2011).
Martin-Belmonte, F. & Perez-Moreno, M. Epithelial cell polarity, stem cells and cancer. Nature Rev. Cancer 12, 23–38 (2010).
Qin, Y., Capaldo, C., Gumbiner, B. M. & Macara, I. G. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 171, 1061–1071 (2005).
Georgiou, M., Marinari, E., Burden, J. & Baum, B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr. Biol. 18, 1631–1638 (2008).
Tsukita, S., Yamazaki, Y., Katsuno, T., Tamura, A. & Tsukita, S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene 27, 6930–6938 (2008).
Dow, L. E. et al. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene 27, 5988–6001 (2008).
Nagasaka, K. et al. The cell polarity regulator hScrib controls ERK activation through a KIM site-dependent interaction. Oncogene 29, 5311–5321 (2010).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Varelas, X. et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell 19, 831–844 (2010).
Osmani, N., Vitale, N., Borg, J.-P. & Etienne-Manneville, S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr. Biol. 16, 2395–2405 (2006).
Dow, L. E. et al. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene 26, 2272–2282 (2007).
Zhan, L. et al. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for polarity in carcinoma. Cell 135, 865–878 (2008).
Nagai-Tamai, Y., Mizuno, K., Hirose, T., Suzuki, A. & Ohno, S. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells 7, 1161–1171 (2002).
Betschinger, J., Mechtler, K. & Knoblich, J. A. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422, 326–330 (2003).
Bilder, D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 18, 1909–1925 (2004).
Gardiol, D., Zacchi, A., Petrera, F., Stanta, G. & Banks, L. Human discs large and scribble are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int. J. Cancer 119, 1285–1290 (2006).
Pearson, H. B. et al. SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. J. Clin. Invest. 121, 4257–4267 (2011).
Caruana, G. & Bernstein, A. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol. Cell. Biol. 21, 1475–1483 (2001).
Klezovitch, O., Fernandez, T. E., Tapscott, S. J. & Vasioukhin, V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 18, 559–571 (2004).
Bouvard, V. et al. A review of human carcinogens-Part B: biological agents. Lancet Oncol. 10, 321–322 (2009).
Bouvard, V. et al. Carcinogenicity of malaria and of some polyomaviruses. Lancet Oncol. 13, 339–340 (2012).
IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Biological Agents Vol. 100B (WHO, 2012).
Moore, P. S. & Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nature Rev. Cancer 10, 878–889 (2010).
Rozenblatt-Rosen, O., et al. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. Nature 487, 491–495 (2012).
Mesri, E. A., Cesarman, E. & Boshoff, C. Kaposi's sarcoma and its associated herpesvirus. Nature Rev. Cancer 10, 707–719 (2010).
Wu, M., Pastor-Pareja, J. C. & Xu, T. Interaction between RasV12 and scribbled clones induces tumour growth and invasion. Nature 463, 545–549 (2010).
Magaldi, T. G. et al. Primary human cervical carcinoma cells require human papillomavirus E6 and E7 expression for ongoing proliferation. Virology 422, 114–124 (2012).
Butz, K. et al. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene 22, 5938–5945 (2003).
Thomas, M. et al. Human papillomaviruses, cervical cancer and cell polarity. Oncogene 27, 7018–7030 (2008).
Barbosa, M. & Schlegel, R. The E6 and E7 genes of HPV-18 are sufficient for inducing two-stage in vitro transformation of human keratinocytes. Oncogene 4, 1529–1532 (1989).
Riley, R. R. et al. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 63, 4862–4871 (2003).
Doorbar, J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. 110, 525–541 (2006).
Moody, C. A. & Laimins, L. A. Human papillomavirus oncoproteins: pathways to transformation. Nature Rev. Cancer 10, 550–560 (2010).
Gardiol, D. et al. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene 18, 5487–5496 (1999).
Nakagawa, S. & Huibregtse, J. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6-AP ubiquitin-protein ligase. Mol. Cell. Biol. 20, 8244–8253 (2000).
Glaunsinger, B., Lee, S. S., Thomas, M., Banks, L. & Javier, R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene 19, 1093–1098 (2000).
Storrs, C. & Silverstein, S. PATJ, a tight junction-associated PDZ protein, is a novel degradation target of high-risk human papillomavirus E6 and the alternatively spliced isoform 18 E6*. J. Virol. 8, 4080–4090 (2007).
Thomas, M., Massimi, P., Navarro, C., Borg, J.-P. & Banks, L. The hScrib/Dlg apico-basal control complex is differentially targeted by HPV-16 and HPV-18 E6 proteins. Oncogene 24, 6222–6230 (2005).
Tomaić, V. et al. Human and primate tumour viruses use PDZ binding as an evolutionarily conserved mechanism of targeting cell polarity regulators. Oncogene 28, 1–8 (2009).
Zhang, Y. et al. Structures of a human papillomavirus (HPV) E6 polypeptide bound to MAGUK proteins: mechanisms of targeting tumor suppressors by a high-risk HPV oncoprotein. J. Virol. 81, 3618–3626 (2007).
Lee, C. & Laimins, L. A. Role of the PDZ domain-binding motif of the oncoprotein E6 in the pathogenesis of human papillomavirus type 31. J. Virol. 78, 12366–12377 (2004).
Nicolaides, L. et al. Stabilization of HPV16 E6 protein by PDZ proteins, and potential implications for genome maintenance. Virology 414, 137–145 (2011).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005).
Poulson, N. D. & Lechler, T. Robust control of mitotic spindle orientation in the developing epidermis. J. Cell Biol. 191, 915–922 (2010).
Hao, Y. Par3 controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. Curr. Biol. 20, 1809–1818 (2010).
Johnson, C. A., Hirono, K., Pehoda, K. E. & Do, C. Q. Identification of an Aurora-A/Pinslinker/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell 138, 1150–1163 (2009).
Knoblich, J. A. Mechanisms of asymmetric stem cell division. Cell 132, 583–597 (2008).
Reuter, J. D., Gomez, D., Brandsma, J. L., Rose, J. K. & Roberts, A. Optimization of cottontail rabbit papilloma virus challenge technique. J. Virol. Methods 98, 127–134 (2001).
O'Neill, A. K. et al. Protein kinase Cα promotes cell migration through a PDZ-dependent interaction with its novel substrate discs large homolog 1 (DLG1). J. Biol. Chem. 286, 43559–43568 (2011).
Montcouquiol, M. et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423, 173–177 (2003).
Matthews, K. et al. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated downregulation of E-cadherin. J. Virol. 77, 8378–8385 (2003).
Laurson, J., Khan, S., Chung, R., Cross, K. & Raj, K. Epigenetic repression of E-cadherin by human papillomavirus 16 E7 protein. DrosophilaCarcinogenesis 31, 918–926 (2010).
Kranjec, C. & Banks, L. A systematic analysis of human papillomavirus (HPV) E6 PDZ substrates identifies MAGI-1 as a major target of HPV type 16 (HPV-16) and HPV-18 whose loss accompanies disruption of tight junctions. J. Virol. 85, 1757–1764 (2011).
Herfs, M. et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc. Natl Acad. Sci. USA 109, 10516–10521 (2012).
Shai, A., Brake, T., Somoza, C. & Lambert, P. F. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 67, 1626–1635 (2007).
Nguyen, M. L., Nguyen, M. M., Lee, D., Griep, A. E. & Lambert, P. F. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6's induction of epithelial hyperplasia in vivo. J. Virol. 77, 6957–6964 (2003).
Watson, R. A., Thomas, M., Banks, L. & Roberts, S. Activity of the human papillomavirus E6 PDZ-binding motif correlates with an enhanced morphological transformation of immortalized human keratinocytes. J. Cell Sci. 116, 4925–4934 (2003).
Watson, R. A. et al. Changes in expression of the human homologue of the Drosophila discs large tumour suppressor in high-grade premalignant cervical neoplasias. Carcinogenesis 23, 1791–1796 (2002).
Jeanes, A., Gottardi, C. J. & Yap, A. S. Cadherins and cancer: how does cadherin dysfunction promote tumour progression. Oncogene 27, 6920–6929 (2008).
Massimi, P., Narayan, N., Cuenda, A. & Banks, L. Phosphorylation of the discs large tumour suppressor protein controls its membrane localisation and enhances its susceptibility to HPV E6− induced degradation. Oncogene 25, 4276–4285 (2006).
Krishna Subbaiah, V. et al. The invasive capacity of HPV transformed cells requires the hDlg-dependent enhancement of SGEF/RhoG activity. PLoS Pathog. 8, e1002543 (2012).
Frese, K. K. et al. Oncogenic function for the Dlg1 mammalian homolog of the Drosophila discs-large tumor suppressor. EMBO J. 25, 1406–1417 (2006).
Bonilla-Delgado, J. et al. The E6 oncoprotein from HPV16 enhances the canonical Wnt/β-catenin pathway in skin epidermis in vivo. Mol. Cancer Res. 10, 250–258 (2012).
Shannon-Lowe, C. & Rowe, M. Epstein-Barr virus infection of polarised epithelial cells via the basolateral surface by memory B cell-mediated transfer infection. PLoS Pathog. 7, e1001338 (2011).
Tsai, C. N., Tsai, C. L., Tse, K. P., Chang, H. Y. & Chang, Y. S. The Epstein-Barr virus oncogene product, latent membrane protein 1, induces the downregulation of E-cadherin gene expression via activation of DNA methyltransferases. Proc. Natl Acad. Sci. USA 99, 10084–10089 (2002).
Scholle, F., Bendt, K. M. & Raab-Traub, N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74, 10681–10689 (2000).
Horikawa, T. et al. Twist and epithelial-mesenchymal transition are induced by the EBV oncoprotein latent membrane protein 1 and are associated with metastatic nasopharyngeal carcinoma. Cancer Res. 67, 1970–1978 (2007).
Horikawa, T. et al. Epstein-Barr Virus latent membrane protein 1 induces Snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. Br. J. Cancer 104, 1160–1167 (2011).
Cano, A. et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biol. 2, 76–83 (2000).
Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004).
Cavatorta, A. L., Giri, A. A., Banks, L. & Gardiol, D. Cloning and functional analysis of the promoter region of the human Disc large gene. Gene 424, 87–95 (2008).
Liu, H. P. et al. Epstein-Barr virus-encoded LMP1 interacts with FGD4 to activate CDC42 and thereby promote migration of nasopharyngeal carcinoma cells. PLoS Pathog. 8, e1002690 (2012).
Shackelford, J., Maier, C. & Pagano, J. S. Epstein-Barr virus activates β-catenin in type III latently infected B lymphocyte lines: association with deubiquitinating enzymes. Proc. Natl Acad. Sci. USA 100, 15572–15576 (2003).
Morrison, J. A. & Raab-Traub, N. Roles of the ITAM & PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of β-catenin signaling. J. Virol. 79, 2375–2382 (2005).
Subbaiah, V. K., Narayan, N., Massimi, P. & Banks, L. Regulation of the DLG tumor suppressor by β-catenin. Int. J. Cancer 131, 2223–2233 (2012).
Fujimuro, M. et al. A novel viral mechanism for dysregulation of β-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nature Med. 9, 300–306 (2003).
Wu, Y. H. et al. The manipulation of miRNA-gene regulatory networks by KSHV induces endothelial motility. Blood 111, 2896–2905 (2011).
Saburi, S. et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nature Genet. 40, 1010–1015 (2008).
Ishiuchi, T., Misaki, K., Yonemura, S., Takeichi, M. & Tanoue, T. Mammalian Fat and Dachsous cadherins regulate apical membrane organization in the embryonic cerebral cortex. J. Cell Biol. 185, 959–967 (2009).
Matakatsu, H. & Blair, S. S. Separating planar cell polarity and Hippo pathway activities of the protocadherins Fat and Dachsous. Development 139, 1498–1508 (2012).
Mansouri, M., Rose, P. P., Moses, A. V. & Früh, K. Remodeling of endothelial adherens junctions by Kaposi's sarcoma-associated herpesvirus. J. Virol. 82, 9615–9628 (2008).
Qian, L. W., Greene, W., Ye, F. & Gao, S. J. Kaposi's sarcoma-associated herpesvirus disrupts adherens junctions and increases endothelial permeability by inducing degradation of VE-cadherin. J. Virol. 82, 11902–11912 (2008).
Dwyer, J. et al. Remodeling of VE-cadherin junctions by the human herpes virus 8 G-protein coupled receptor. Oncogene 30, 190–200 (2011).
Guilluy, C. et al. Latent KSHV infection increases the vascular permeability of human endothelial cells. Blood 118, 5344–5354 (2011).
Satou, Y. et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 7, e1001274 (2011).
Lee, S. S., Weiss, R. S. & Javier, R. T. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc. Natl Acad. Sci. USA 94, 6670–6675 (1997).
Arpin-André, C. & Mesnard, J. M. The PDZ domain-binding motif of the human T cell leukemia virus type 1 tax protein induces mislocalization of the tumor suppressor hScrib in T cells. J. Biol. Chem. 282, 33132–33141 (2007).
Ohashi, M. et al. Human T-cell leukemia virus type 1 Tax oncoprotein induces and interacts with a multi-PDZ domain protein, MAGI-3. Virology 320, 52–62 (2004).
Rousset, R., Fabre, S., Desbois, C., Bantignies, F. & Jalinot, P. The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. Oncogene 16, 643–654 (1998).
Ma, G., Yasunaga, J., Fan, J., Yanagawa, S. & Matsuoka, M. HTLV-1 bZIP factor dysregulates the Wnt pathways to support proliferation and migration of adult T-cell leukemia cells. Oncogene 8 Oct 2012 (doi:10.1038/onc.2012.450).
Higuchi, M. et al. Cooperation of NF-κB2/p100 activation and the PDZ domain binding motif signal in human T-cell leukemia virus type 1 (HTLV-1) Tax1 but not HTLV-2 Tax2 is crucial for interleukin-2-independent growth transformation of a T-cell line. J. Virol. 81, 11900–11907 (2007).
James, M. A., Lee, J. H. & Klingelhutz, A. J. Human papillomavirus type 16 E6 activates NF-κB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J. Virol. 80, 5301–5307 (2006).
Blot, V. et al. Human Dlg protein binds to the envelope glycoproteins of human T-cell leukemia virus type 1 and regulates envelope mediated cell-cell fusion in T lymphocytes. J. Cell Sci. 117, 3983–3993 (2004).
Tanaka, Y., Fukudome, K., Hayashi, M., Takagi, S. & Yoshie, O. Induction of ICAM-1 and LFA-3 by Tax1 of human T-cell leukemia virus type 1 and mechanism of down-regulation of ICAM-1 or LFA-1 in adult-T-cell-leukemia cell lines. Int. J. Cancer 60, 554–561 (1995).
Nejmeddine, M. et al. HTLV-1-Tax and ICAM-1 act on T-cell signal pathways to polarize the microtubule-organizing center at the virological synapse. Blood 114, 1016–1025 (2009).
Hawkins, E. D. & Russell, S. M. Upsides and downsides to polarity and asymmetric cell division in leukemia. Oncogene 27, 7003–7017 (2008).
Renkema, G. H., Manninen, A. & Saksela, K. Human immunodeficiency virus type 1 Nef selectively associates with a catalytically active subpopulation of p21-activated kinase (PAK2) independently of PAK2 binding to Nck or β-PIX. J. Virol. 75, 2154–2160 (2001).
Stevenson, M. HIV-1 pathogenesis. Nature Med. 9, 853–860 (2003).
Margottin, F. et al. A novel human WD protein, h-β-Trcp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1, 565–574 (1998).
Besnard-Guerin, C. et al. HIV-1 Vpu sequesters β-transducin repeat-containing protein (b-Trcp) in the cytoplasm and provokes the accumulation of β-catenin and other SCFb-Trcp substrates. J. Biol. Chem. 279, 788–795 (2004).
Salim, A. & Ratner, L. Modulation of β-catenin and E-cadherin interaction by Vpu increases human immunodeficiency virus type 1 particle release. J. Virol. 82, 3932–3938 (2008).
Pu, H. et al. HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J. Cereb. Blood Flow Metab. 25, 1325–1335 (2005).
Nazli, A. et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 6, e1000852 (2010).
Perrault, M. & Pécheur, E. I. The hepatitis C virus and its hepatic environment: a toxic but finely tuned partnership. Biochem. J. 423, 303–314 (2009).
Lara-Pezzi, E., Roche, S., Andrisani, O. M., Sánchez-Madrid, F. & López-Cabrera, M. The hepatitis B virus HBx protein induces adherens junction disruption in a src-dependent manner. Oncogene 20, 3323–3331 (2001).
Lee, J. O. et al. Hepatitis B virus X protein represses E-cadherin expression via activation of DNA methyltransferase 1. Oncogene 24, 6617–6625 (2005).
Arzumanyan, A. et al. Epigenetic repression of E-cadherin expression by hepatitis B virus x antigen in liver cancer. Oncogene 31, 563–572 (2012).
Hsieh, A., Kim, H. S., Lim, S. O., Yu, D. Y. & Jung, G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/β-catenin signaling. Cancer Lett. 300, 162–172 (2011).
Zhang, T. et al. Hepatitis B virus X protein (HBx) modulates oncogene YAP via CREB to promote growth of hepatoma cells. Hepatology 18 Jun 2012 (doi:10.1002/hep.25899).
Tsai, W. L. & Chung, R. T. Viral hepatocarcinogenesis. Oncogene 29, 2309–2324 (2010).
Arora, P., Kim, E. O., Jung, J. K. & Jang, K. L. Hepatitis C virus core protein downregulates E-cadherin expression via activation of DNA methyltransferase 1 and 3b. Cancer Lett. 261, 244–252 (2008).
Street, A., Macdonald, A., McCormick, C. & Harris, M. Hepatitis C virus NS5A-mediated activation of phosphoinositide 3-kinase results in stabilization of cellular β-catenin and stimulation of β-catenin-responsive transcription. J. Virol. 79, 5006–5016 (2005).
Akkari, L. et al. Hepatitis C viral protein NS5A induces EMT and participates in oncogenic transformation of primary hepatocyte precursors. J. Hepatol. 57, 1021–1028 (2012).
Battaglia, S. et al. Liver cancer-derived hepatitis C virus core proteins shift TGF-β responses from tumor suppression to epithelial-mesenchymal transition. PLoS ONE 4, e4355 (2009).
Mee, C. J. et al. Effect of cell polarization on hepatitis C virus entry. J. Virol. 82, 461–470 (2008).
Benedicto, I. et al. The tight junction-associated protein occludin is required for a postbinding step in hepatitis C virus entry and infection. J. Virol. 83, 8012–8020 (2009).
Liu, S. et al. Tight junction proteins claudin-1 and occludin control hepatitis C virus entry and are downregulated during infection to prevent. superinfection. J. Virol. 83, 2011–2014 (2009).
Feng, H., Shuda, M., Chang, Y. & Moore, P. S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319, 1096–1100 (2008).
Shuda, M. et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc. Natl Acad. Sci. USA 105, 16272–16277 (2008).
Kirschner, N. et al. CD44 regulates tight-junction assembly and barrier function. J. Invest. Dermatol. 131, 932–943 (2011).
Meinke, P., Nguyen, T. D. & Wehnert, M. S. The LINC complex and human disease. Biochem. Soc. Trans. 39, 1693–1697 (2011).
Markiewicz, E. et al. The inner nuclear membrane protein emerin regulates β-catenin activity by restricting its accumulation in the nucleus. EMBO J. 25, 3275–3285 (2006).
Cheng, J., DeCaprio, J. A., Fluck, M. M. & Schaffhausen, B. S. Cellular transformation by Simian Virus 40 and Murine Polyoma Virus T antigens. Semin. Cancer Biol. 19, 218–228 (2009).
Tian, Y., Li, D., Dahl, J., You, J. & Benjamin, T. Identification of TAZ as a binding partner of the polyomavirus T antigens. J. Virol. 78, 12657–12664 (2004).
Acknowledgements
L.B. gratefully acknowledges research support provided by the Associazione Italiana per la Ricerca sul Cancro, Telethon Grant GGP10006 and the Wellcome Trust. This article is dedicated to the memory of Joan Banks and John Pim.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Apico-basal polarity
-
(ABP). The vertical orientation of a cell, defined and maintained by functional differences and molecular gradients between the apical and basal parts of the cell; vital in epithelia.
- Cadherins
-
Transmembrane proteins involved in cell–cell adhesion, often disrupted or altered during tumorigenesis.
- E-cadherin
-
The prototype cadherin in epithelial cells, part of the adherens junction, thus contributing to ABP; thought to sequester β-catenin, thus inhibiting its activation of the canonical WNT signalling pathway.
- FAT4 cadherin
-
A massive cadherin-related protein, homologue of Drosophila melanogaster Fat tumour suppressor, which functions upstream of the Hippo pathway in regulating PCP and organ size.
- Immune synapse
-
Interface between a naive cytotoxic T-lymphocyte (CTL) and an antigen-presenting cell, activating a pathway that results in changes in actin polymerization and cell morphology necessary for CTL maturation.
- Keratinocytes
-
The major cells (∼95%) of the epidermis, which differentiate upwards from the basement membrane, gradually losing nuclei and expressing massive amounts of keratins to form the impermeable skin layer, which is shed by desquamation.
- Merkel cell
-
A cell in the epithelium that is essential for the fine resolution of sensory stimuli; malignantly transformed in Merkel cell carcinoma.
- Organotypic raft cultures
-
A tissue culture method of growing keratinocytes at the liquid–air interface to recapitulate epithelial differentiation in vitro.
- Planar cell polarity
-
(PCP). The horizontal organization of a cell, such that specialized structures (for example, adherens junctions) are orientated in the same plane of the epithelium; also, the polarization of cells within the plane of a tissue.
- VE-cadherin
-
An endothelial cell-specific cadherin that regulates vascular morphology and stability and is instrumental in regulating PCP.
- Virological synapse
-
A virus-induced interface between infected and uninfected cells, allowing efficient cell-to-cell spread of virus.
Rights and permissions
About this article
Cite this article
Banks, L., Pim, D. & Thomas, M. Human tumour viruses and the deregulation of cell polarity in cancer. Nat Rev Cancer 12, 877–886 (2012). https://doi.org/10.1038/nrc3400
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3400
This article is cited by
-
Novel effect of the high risk-HPV E7 CKII phospho-acceptor site on polarity protein expression
BMC Cancer (2022)
-
Quantitative fragmentomics allow affinity mapping of interactomes
Nature Communications (2022)
-
Microbial Signatures Associated with Oropharyngeal and Oral Squamous Cell Carcinomas
Scientific Reports (2017)
-
Regulation of aPKC activity by Nup358 dependent SUMO modification
Scientific Reports (2016)
-
Par6G suppresses cell proliferation and is targeted by loss-of-function mutations in multiple cancers
Oncogene (2016)