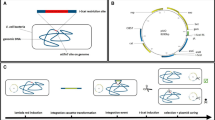
Abstract
Full-length RecE and RecT from Rac prophage mediate highly efficient linear–linear homologous recombination that can be used to clone large DNA regions directly from genomic DNA into expression vectors, bypassing library construction and screening. Homologous recombination mediated by Redαβ from lambda phage has been widely used for recombinant DNA engineering. Here we present a protocol for direct cloning and engineering of biosynthetic gene clusters, large operons or single genes from genomic DNA using one Escherichia coli host that harbors both RecET and Redαβ systems. The pipeline uses standardized cassettes for horizontal gene transfer options, as well as vectors with different replication origins configured to minimize recombineering background through the use of selectively replicating templates or CcdB counterselection. These optimized reagents and protocols facilitate fast acquisition of transgenes from genomic DNA preparations, which are ready for heterologous expression within 1 week.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Van Lanen, S.G. & Shen, B. Microbial genomics for the improvement of natural product discovery. Curr. Opin. Microbiol. 9, 252–260 (2006).
Nett, M., Ikeda, H. & Moore, B.S. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 26, 1362–1384 (2009).
Lautru, S., Deeth, R.J., Bailey, L.M. & Challis, G.L. Discovery of a new peptide natural product by Streptomyces coelicolor genome mining. Nat. Chem. Biol. 1, 265–269 (2005).
Bergmann, S. et al. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 3, 213–217 (2007).
Wenzel, S.C. & Müller, R. The impact of genomics on the exploitation of the myxobacterial secondary metabolome. Nat. Prod. Rep. 26, 1385–1407 (2009).
Charlop-Powers, Z., Milshteyn, A. & Brady, S.F. Metagenomic small molecule discovery methods. Curr. Opin. Microbiol. 19, 70–75 (2014).
Fu, J. et al. Full-length RecE enhances linear-linear homologous recombination and facilitates direct cloning for bioprospecting. Nat. Biotechnol. 30, 440–446 (2012).
Yamanaka, K. et al. Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc. Natl. Acad. Sci. USA 111, 1957–1962 (2014).
Tang, Y. et al. Heterologous expression of an orphan NRPS gene cluster from Paenibacillus larvae in Escherichia coli revealed production of sevadicin. J. Biotechnol. 194, 112–114 (2015).
Yin, W.B. et al. Discovery of cryptic polyketide metabolites from dermatophytes using heterologous expression in Aspergillus nidulans. ACS Synth. Biol. 2, 629–634 (2013).
Wolpert, M., Heide, L., Kammerer, B. & Gust, B. Assembly and heterologous expression of the coumermycin A1 gene cluster and production of new derivatives by genetic engineering. Chembiochem 9, 603–612 (2008).
Chai, Y. et al. Heterologous expression and genetic engineering of the tubulysin biosynthetic gene cluster using Red/ET recombineering and inactivation mutagenesis. Chem. Biol. 19, 361–371 (2012).
Fu, J. et al. Efficient transfer of two large secondary metabolite pathway gene clusters into heterologous hosts by transposition. Nucleic Acids Res. 36, e113 (2008).
Perlova, O. et al. Reconstitution of the myxothiazol biosynthetic gene cluster by Red/ET recombination and heterologous expression in Myxococcus xanthus. Appl. Environ. Microbiol. 72, 7485–7494 (2006).
Wenzel, S.C. et al. Heterologous expression of a myxobacterial natural products assembly line in pseudomonads via red/ET recombineering. Chem. Biol. 12, 349–356 (2005).
Wang, H. et al. Improved seamless mutagenesis by recombineering using ccdB for counterselection. Nucleic Acids Res. 42, e37 (2014).
Zhang, Y., Buchholz, F., Muyrers, J.P. & Stewart, A.F. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 20, 123–128 (1998).
Zhang, Y., Muyrers, J.P., Testa, G. & Stewart, A.F. DNA cloning by homologous recombination in Escherichia coli. Nat. Biotechnol. 18, 1314–1317 (2000).
Bian, X. et al. Direct cloning, genetic engineering, and heterologous expression of the syringolin biosynthetic gene cluster in E. coli through Red/ET recombineering. Chembiochem 13, 1946–1952 (2012).
Bian, X. et al. Heterologous production of glidobactins/luminmycins in Escherichia coli Nissle containing the glidobactin biosynthetic gene cluster from Burkholderia DSM7029. Chembiochem 15, 2221–2224 (2014).
Bian, X. et al. In vivo evidence for a prodrug activation mechanism during colibactin maturation. Chembiochem 14, 1194–1197 (2013).
Kouprina, N. & Larionov, V. Selective isolation of genomic loci from complex genomes by transformation-associated recombination cloning in the yeast Saccharomyces cerevisiae. Nat. Protoc. 3, 371–377 (2008).
Shao, Z. & Zhao, H. DNA assembler, an in vivo genetic method for rapid construction of biochemical pathways. Nucleic Acids Res. 37, e16 (2009).
Gibson, D.G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Muyrers, J.P., Zhang, Y., Testa, G. & Stewart, A.F. Rapid modification of bacterial artificial chromosomes by ET-recombination. Nucleic Acids Res. 27, 1555–1557 (1999).
Muyrers, J.P. et al. Point mutation of bacterial artificial chromosomes by ET recombination. EMBO Rep. 1, 239–243 (2000).
Eustaquio, A.S. et al. Heterologous expression of novobiocin and clorobiocin biosynthetic gene clusters. Appl. Environ. Microbiol. 71, 2452–2459 (2005).
Binz, T.M., Wenzel, S.C., Schnell, H.J., Bechthold, A. & Müller, R. Heterologous expression and genetic engineering of the phenalinolactone biosynthetic gene cluster by using red/ET recombineering. Chembiochem 9, 447–454 (2008).
Ongley, S.E. et al. High-titer heterologous production in E. coli of lyngbyatoxin, a protein kinase C activator from an uncultured marine cyanobacterium. ACS Chem. Biol. 8, 1888–1893 (2013).
Tang, L. et al. Elucidating the mechanism of cis double bond formation in epothilone biosynthesis. J. Am. Chem. Soc. 126, 46–47 (2004).
Bian, X., Plaza, A., Yan, F., Zhang, Y. & Müller, R. Rational and efficient site-directed mutagenesis of adenylation domain alters relative yields of luminmide derivatives in vivo. Biotechnol. Bioeng. 112, 1343–1353 (2015).
Nguyen, K.T. et al. Genetically engineered lipopeptide antibiotics related to A54145 and daptomycin with improved properties. Antimicrob. Agents Chemother. 54, 1404–1413 (2010).
Nguyen, K.T. et al. Combinatorial biosynthesis of novel antibiotics related to daptomycin. Proc. Natl. Acad. Sci. USA 103, 17462–17467 (2006).
Alexander, D.C. et al. Development of a genetic system for combinatorial biosynthesis of lipopeptides in Streptomyces fradiae and heterologous expression of the A54145 biosynthesis gene cluster. Appl. Environ. Microbiol. 76, 6877–6887 (2010).
Yin, J. et al. Direct cloning and heterologous expression of the salinomycin biosynthetic gene cluster from Streptomyces albus DSM41398 in Streptomyces coelicolor A3(2). Sci. Rep. 5, 15081 (2015).
Wang, J. et al. An improved recombineering approach by adding RecA to lambda Red recombination. Mol. Biotechnol. 32, 43–53 (2006).
Sarov, M. et al. A recombineering pipeline for functional genomics applied to Caenorhabditis elegans. Nat. Methods 3, 839–844 (2006).
Filutowicz, M., McEachern, M.J. & Helinski, D.R. Positive and negative roles of an initiator protein at an origin of replication. Proc. Natl. Acad. Sci. USA 83, 9645–9649 (1986).
Sorensen, H.P. & Mortensen, K.K. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J. Biotechnol. 115, 113–128 (2005).
Li, Y. et al. Directed natural product biosynthesis gene cluster capture and expression in the model bacterium Bacillus subtilis. Sci. Rep. 5, 9383 (2015).
Frengen, E. et al. A modular, positive selection bacterial artificial chromosome vector with multiple cloning sites. Genomics 58, 250–253 (1999).
Mei, J., Benashski, S. & Firshein, W. Interactions of the origin of replication (oriV) and initiation proteins (TrfA) of plasmid RK2 with submembrane domains of Escherichia coli. J. Bacteriol. 177, 6766–6772 (1995).
Nedelkova, M. et al. Targeted isolation of cloned genomic regions by recombineering for haplotype phasing and isogenic targeting. Nucleic Acids Res. 39, e137 (2011).
Lynch, M.D. & Gill, R.T. Broad host range vectors for stable genomic library construction. Biotechnol. Bioeng. 94, 151–158 (2006).
Antoine, R. & Locht, C. Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from gram-positive organisms. Mol. Microbiol. 6, 1785–1799 (1992).
Bi, C. et al. Development of a broad-host synthetic biology toolbox for Ralstonia eutropha and its application to engineering hydrocarbon biofuel production. Microb. Cell Fact. 12, 107 (2013).
Acknowledgements
This work was supported by the International ST Cooperation Program of China (ISTCP 2015DFE32850 to J.F.), a Shandong Innovation and Transformation of Achievements Grant (2014ZZCX02601 to Y.Z.) and a Shenzhen Global Experts Innovation and Pioneering Grant (KQCX20120806161031208 to J.F.). Y.Z. is supported by the Recruitment Program of Global Experts in Shandong University. H.W. is supported by the China Postdoctoral Science Foundation (2015T80710) and the Postdoctoral Innovation Program of Shandong Province (201303110). A.F.S. is supported by the TUD Elite University Support the Best program. The authors acknowledge V. Ravichandir's help in proofreading this manuscript.
Author information
Authors and Affiliations
Contributions
H.W., Z.L., R.M., A.F.S., J.F. and Y.Z. designed the experiments. H.W., Z.L., R.J., Y.H., J.Y., X.B. and A.L. performed the experiments. H.W., A.F.S., J.F. and Y.Z. wrote the manuscript with help from all authors.
Corresponding authors
Ethics declarations
Competing interests
R.M., A.F.S. and Y.Z. are shareholders in Gene Bridges GmbH, which holds exclusive rights to the commercialization of both Redαβ and RecET recombineering technologies.
Integrated supplementary information
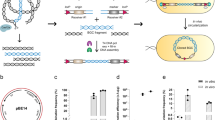
Supplementary Figure 1 Derivation of pBAC2015.
(a) pBAC2015 was derived from pBACe3.6 by insertion of oriV and removal of a 30 bp repeat and the loxP site. (b) Comparison of direct cloning with pBACe3.6 and pBAC2015. Homology arms to the 37.5 kb Photorhabdus luminescens plu3535-plu3532 gene cluster were added using the method illustrated in Supplementary Figure 2. Most of the colonies obtained with pBACe3.6 were due to intramolecular recombination through the 30 bp repeat. Its removal in pBAC2015 dramatically reduced the unwanted background so that most colonies contained the intended product.
Supplementary Figure 2 Construction of linear BAC direct cloning vectors.
(a) The cassette containing pBR322, amp and ccdB is amplified by two rounds of PCR to attach homology arms to the gene cluster (ha1 and ha2) and pBAC2015 (ha3 and ha4) respectively. (b) The cassette containing pBR322, amp and ccdB is recombined with pBAC2015 by LLHR in RecET expressing CcdB resistant strain, E. coli GBdir-gyrA462. Because the pBR322-amp-ccdB-rpsL plasmid carries the rpsL gene, in addition to ampicillin and chloramphenicol selection, streptomycin selection can be added to eliminate carryover background from the PCR template. The BAC vector is linearized by BamHI digestion to expose the terminal homology arms.
Supplementary Figure 3 Optimization of R6K plasmids with transposition cassette for different hosts.
(a) pR6K-oriT-tnpA-genta is the same as pR6K-oriT-tnpA-kan except the gentamicin resistance gene replaced the kanamycin resistance gene. (b) pR6K-oriT-tnpA-apra is the same as pR6K-oriT-tnpA-kan except for the inclusion of the apramycin resistance gene and the NcoI site. (c) Exchanging the promoter of tnpA via LLHR. Electroporate the BstZ17I digested pR6K-oriT-TnpA-kan plasmid together with a synthetic oligonucleotide, a synthetic DNA or a PCR product into RecET expressing pir strain, E. coli GBdir-pir116, for LLHR. In case of using synthetic oligonucleotides, the homology arms can be as short as 20 nt to ensure the oligonucleotide is not longer than 140 nt.
Supplementary information
Supplementary Information
Supplementary Figures 1–3, Supplementary Tables 1–3 and Supplementary Notes 1–10 (PDF 764 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Li, Z., Jia, R. et al. RecET direct cloning and Redαβ recombineering of biosynthetic gene clusters, large operons or single genes for heterologous expression. Nat Protoc 11, 1175–1190 (2016). https://doi.org/10.1038/nprot.2016.054
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.054
This article is cited by
-
Rapid Rescue of Goose Astrovirus Genome via Red/ET Assembly
Food and Environmental Virology (2024)
-
Improving spinosad production by tuning expressions of the forosamine methyltransferase and the forosaminyl transferase to reduce undesired less active byproducts in the heterologous host Streptomyces albus J1074
Microbial Cell Factories (2023)
-
Systematic identification of endogenous strong constitutive promoters from the diazotrophic rhizosphere bacterium Pseudomonas stutzeri DSM4166 to improve its nitrogenase activity
Microbial Cell Factories (2023)
-
Precise genome engineering in Pseudomonas using phage-encoded homologous recombination and the Cascade–Cas3 system
Nature Protocols (2023)
-
Complete Genome Sequence of Streptomyces sp. HP-A2021, a Promising Bacterium for Natural Product Discovery
Biochemical Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.