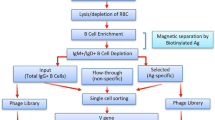
Abstract
Predicting immune responses before vaccination is challenging because of the complexity of the governing parameters. Nevertheless, recent work has shown that B cell receptor (BCR)-antigen engagement in vitro can prove a powerful means of informing the design of antibody-based vaccines. We have developed this principle into a two-phased immunogen evaluation pipeline to rank-order vaccine candidates. In phase 1, recombinant antigens are screened for reactivity to the germline precursors that produce the antibody responses of interest. To both mimic the architecture of initial antigen engagement and facilitate rapid immunogen screening, these antibodies are expressed as membrane-anchored IgM (mIgM) in 293F indicator cells. In phase 2, the binding hits are multimerized by nanoparticle or proteoliposome display, and they are evaluated for BCR triggering in an engineered B cell line displaying the IgM sequences of interest. Key developments that complement existing methodology in this area include the following: (i) introduction of a high-throughput screening step before evaluation of more time-intensive BCR-triggering analyses; (ii) generalizable multivalent antigen-display platforms needed for BCR activation; and (iii) engineered use of a human B cell line that does not display endogenous antibody, but only ectopically expressed BCR sequences of interest. Through this pipeline, the capacity to initiate favorable antibody responses is evaluated. The entire protocol can be completed within 2.5 months.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
McHeyzer-Williams, L.J. & McHeyzer-Williams, M.G. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23, 487–513 (2005).
Slifka, M.K. & Amanna, I. How advances in immunology provide insight into improving vaccine efficacy. Vaccine 32, 2948–2957 (2014).
Vinuesa, C.G., Linterman, M.A., Goodnow, C.C. & Randall, K.L. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol. Rev. 237, 72–89 (2010).
G Gruell, H. & Klein, F. Opening fronts in HIV vaccine development: tracking the development of broadly neutralizing antibodies. Nat. Med. 20, 478–479 (2014).
Kirchenbaum, G.A. & Ross, T.M. Eliciting broadly protective antibody responses against influenza. Curr. Opin. Immunol. 28C, 71–76 (2014).
Lingwood, D. et al. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature 489, 566–570 (2012).
Jardine, J. et al. Rational HIV immunogen design to target specific germline B cell receptors. Science 340, 711–716 (2013).
Ota, T. et al. Anti-HIV B cell lines as candidate vaccine biosensors. J. Immunol. 189, 4816–4824 (2012).
McGuire, A.T. et al. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J. Exp. Med. 210, 655–663 (2013).
Hoot, S. et al. Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs. PLoS Pathog. 9, e1003106 (2013).
McGuire, A.T., Glenn, J.A., Lippy, A. & Stamatatos, L. Diverse recombinant HIV-1 Envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D. J. Virol. 88, 2645–2657 (2014).
McGuire, A.T. et al. HIV antibodies. Antigen modification regulates competition of broad and narrow neutralizing HIV antibodies. Science 346, 1380–1383 (2014).
Thomson, C.A. et al. Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front. Immunol. 3, 87 (2012).
Corti, D. et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 120, 1663–1673 (2010).
Wrammert, J. et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208, 181–193 (2011).
Avnir, Y. et al. Molecular signatures of hemagglutinin stem-directed heterosubtypic human neutralizing antibodies against influenza A viruses. PLoS Pathog. 10, e1004103 (2014).
Pappas, L. et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature 516, 418–422 (2014).
Wheatley, A.K. et al. H5N1 vaccine-elicited memory B cells are genetically constrained by the IGHV locus in the recognition of a neutralizing epitope in the hemagglutinin stem. J. Immunol. 195, 602–610 (2015).
Whittle, J.R. et al. Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy-chain lineages. J. Virol. 88, 4047–4057 (2014).
Zhou, T. et al. Structural repertoire of HIV-1-neutralizing antibodies targeting the CD4 supersite in 14 donors. Cell 161, 1280 1292 (2015).
Dosenovic, P. et al. Immunization for HIV-1 broadly neutralizing antibodies in human Ig knock-in mice. Cell 161, 1505–1515 (2015).
Jardine, J.G. et al. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 349, 156–161 (2015).
Yassine, H.M. et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 21, 1065–1070 (2015).
Davey, A.M. & Pierce, S.K. Intrinsic differences in the initiation of B cell receptor signaling favor responses of human IgG+ memory B cells over IgM+ naive B cells. J. Immunol. 188, 3332–3341 (2012).
Xu, Y. et al. No receptor stands alone: IgG B-cell receptor intrinsic and extrinsic mechanisms contribute to antibody memory. Cell Res. 24, 651–664 (2014).
Kanekiyo, M. et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499, 102–106 (2013).
Metzger, H. Transmembrane signaling: the joy of aggregation. J. Immunol. 149, 1477–1487 (1992).
Akahata, W. et al. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat. Med. 16, 334–338 (2010).
Cumbers, S.J. et al. Generation and iterative affinity maturation of antibodies in vitro using hypermutating B-cell lines. Nat. Biotechnol. 20, 1129–1134 (2002).
Novak, E.J. & Rabinovitch, P.S. Improved sensitivity in flow cytometric intracellular ionized calcium measurement using fluo-3/fura red fluorescence ratios. Cytometry 17, 135–141 (1994).
Wendt, E.R., Ferry, H., Greaves, D.R. & Keshav, S. Ratiometric analysis of fura red by flow cytometry: a technique for monitoring intracellular calcium flux in primary cell subsets. PLoS ONE 10, e0119532 (2015).
Dintzis, H.M., Dintzis, R.Z. & Vogelstein, B. Molecular determinants of immunogenicity: the immunon model of immune response. Proc. Natl. Acad. Sci. USA 73, 3671–3675 (1976).
Luo, X.M. et al. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood 113, 1422–1431 (2009).
Bachmann, M.F. & Jennings, G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796 (2010).
Batista, F.D. & Harwood, N.E. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 9, 15–27 (2009).
Cyster, J.G. B cell follicles and antigen encounters of the third kind. Nat. Immunol. 11, 989–996 (2010).
Lois, C., Hong, E.J., Pease, S., Brown, E.J. & Baltimore, D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872 (2002).
Naldini, L., Blomer, U., Gage, F.H., Trono, D. & Verma, I.M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. USA 93, 11382–11388 (1996).
Schroeder, H.W. Jr. & Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 125, S41–S52 (2010).
Wu, X. et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333, 1593–1602 (2011).
Kabat, E.A., Wu, T.T., Perry, H.M., Gottesman, K.S. & Foeller, C. Sequences of Proteins of Immunological Interest (U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, 1991).
Lefranc, M.P. et al. IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev. Comp. Immunol. 27, 55–77 (2003).
Kong, W.P. et al. Protective immunity to lethal challenge of the 1918 pandemic influenza virus by vaccination. Proc. Natl. Acad. Sci. USA 103, 15987–15991 (2006).
Wei, C.J. et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329, 1060–1064 (2010).
Muller, R., Wienands, J. & Reth, M. The serine and threonine residues in the Ig-α cytoplasmic tail negatively regulate immunoreceptor tyrosine-based activation motif-mediated signal transduction. Proc. Natl. Acad. Sci. USA 97, 8451–8454 (2000).
Heizmann, B., Reth, M. & Infantino, S. Syk is a dual-specificity kinase that self-regulates the signal output from the B-cell antigen receptor. Proc. Natl. Acad. Sci. USA 107, 18563–18568 (2010).
Sulzer, B. & Perelson, A.S. Immunons revisited: binding of multivalent antigens to B cells. Mol. Immunol. 34, 63–74 (1997).
Ekiert, D.C. et al. Antibody recognition of a highly conserved influenza virus epitope. Science 324, 246–251 (2009).
Acknowledgements
The authors thank members of the Lingwood laboratory for critical reading of the manuscript, S. Perfetto (Vaccine Research Center, National Institutes of Health (NIH)) for flow cytometry advice, and J. Boyington and J. Whittle (Vaccine Research Center, NIH) for key discussions. This work was supported by the following awards to D.L.: a Harvard University Center for AIDS Research (CFAR) grant (P30 AI060354); a Broad-Ragon ENDHIV Catalytic grant; the William F. Milton Fund; and the Gilead Sciences Research Scholars Program in HIV.
Author information
Authors and Affiliations
Contributions
G.C.W., R.F.V., M.K., G.J.N., J.R.M. and D.L. designed the research; G.C.W., R.F.V., M.K. and D.L. performed the research; G.C.W., R.F.V., M.K., G.J.N., J.R.M. and D.L. analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Weaver, G., Villar, R., Kanekiyo, M. et al. In vitro reconstitution of B cell receptor–antigen interactions to evaluate potential vaccine candidates. Nat Protoc 11, 193–213 (2016). https://doi.org/10.1038/nprot.2016.009
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.009
This article is cited by
-
Enhancing antibody responses by multivalent antigen display on thymus-independent DNA origami scaffolds
Nature Communications (2024)
-
Engaging an HIV vaccine target through the acquisition of low B cell affinity
Nature Communications (2023)
-
A highly immunogenic vaccine platform against encapsulated pathogens using chimeric probiotic Escherichia coli membrane vesicles
npj Vaccines (2022)
-
Role of nanoscale antigen organization on B-cell activation probed using DNA origami
Nature Nanotechnology (2020)
-
Engineered immunogen binding to alum adjuvant enhances humoral immunity
Nature Medicine (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.