Abstract
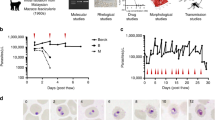
This protocol describes a method for preparing cultures of Plasmodium falciparum synchronized at any intraerythrocytic stage. Using this method, around 60% parasitized cells may be obtained. On the basis of Trager and Jensen's original continuous culture method, our approach relies on the use of fresh human blood not older than 2 weeks, a low hematocrit between 0.8 and 1.5%, a starting frozen inoculum of 10% ring-stage parasitemia, human serum replaced with AlbuMAX I and alternating sorbitol and Percoll synchronization methods to shorten the cycle window to 4–6 h and reduce sorbitol toxicity. From our synchronized high parasite density cultures, 3–5 ml of infected red blood cells can be obtained in 1 week, corresponding to 1.2 mg of total parasite protein per ml of harvested culture. On the basis of the variables parasitemia and packed cell volume, we provide an equation to accurately calculate the amount of complete medium required every 24 h corrected for the cycle stage and capacity of the culture flask. Ten days suffice to complete the protocol from a frozen stock of parasites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schuster, F.L. Cultivation of Plasmodium spp. Clin. Microbiol. Rev. 15, 355–364 (2002).
Ferrer, J. et al. Effect of the haematocrit layer geometry on Plasmodium falciparum static thin-layer in vitro cultures. Malar. J. 7, 203 (2008).
Orjih, A.U. Requirements for maximal enrichment of viable intraerythrocytic Plasmodium falciparum rings by saponin hemolysis. Exp. Biol. Med. (Maywood) 233, 1359–1367 (2008).
Trager, W. & Jensen, J.B. Human malaria parasites in continuous culture. Science 193, 673–675 (1976).
Trager, W. & Jensen, J.B. Continuous culture of Plasmodium falciparum: its impact on malaria research. Int. J. Parasitol. 27, 989–1006 (1997).
Divo, A.A., Vande Waa, J.A., Campbell, J.R. & Jensen, J.B. Isolation and cultivation of Plasmodium falciparum using adult bovine serum. J. Parasitol. 71, 504–509 (1985).
Trager, W. & Jensen, J.B. Cultivation of erythrocytic stages. Bull. World Health Organ. 55, 363–365 (1977).
Lingnau, A., Margos, G., Maier, W.A. & Seitz, H.M. Serum-free cultivation of several Plasmodium falciparum strains. Parasitol. Res. 80, 84–86 (1994).
Flores, M.V., Berger-Eiszele, S.M. & Stewart, T.S. Long-term cultivation of Plasmodium falciparum in media with commercial non-serum supplements. Parasitol. Res. 83, 734–736 (1997).
Chin, W., Moss, D. & Collins, W.E. The continuous cultivation of Plasmodium fragile by the method of Trager–Jensen. Am. J. Trop. Med. Hyg. 28, 591–592 (1979).
Binh, V.Q., Luty, A.J. & Kremsner, P.G. Differential effects of human serum and cells on the growth of Plasmodium falciparum adapted to serum-free in vitro culture conditions. Am. J. Trop. Med. Hyg. 57, 594–600 (1997).
Zolg, J.W., MacLeod, A.J., Dickson, I.H. & Scaife, J.G. Plasmodium falciparum: modifications of the in vitro culture conditions improving parasitic yields. J. Parasitol. 68, 1072–1080 (1982).
Singh, K., Agarwal, A., Khan, S.I., Walker, L.A. & Tekwani, B.L. Growth, drug susceptibility, and gene expression profiling of Plasmodium falciparum cultured in medium supplemented with human serum or lipid-rich bovine serum albumin [corrected]. J. Biomol. Screen. 12, 1109–1114 (2007).
Trager, W. & Jensen, J.B. Cultivation of malarial parasites. Nature 273, 621–622 (1978).
Jensen, J.B. In vitro culture of Plasmodium falciparum parasites. in Malaria Methods and Protocols (ed. Doolan, D.L.) 477–488 (Humana Press, Totowa, New Jersey, 2002).
Raventos-Suarez, C. Plasmodium falciparum: invasion and development in highly parasitized cultures. In Vitro Cell Dev. Biol. 21, 161–164 (1985).
Vernot, J.P. & Wasserman, M. Plasmodium falciparum increased and multiple invasion during short periods of time. J. Protozool. 37, 47–49 (1990).
Radfar, A., Diez, A. & Bautista, J.M. Chloroquine mediates specific proteome oxidative damage across the erythrocytic cycle of resistant Plasmodium falciparum . Free Radic. Biol. Med. 44, 2034–2042 (2008).
Gardner, M.J. et al. Genome sequence of the human malaria parasite Plasmodium falciparum . Nature 419, 498–511 (2002).
Ley, T.J. et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 456, 66–72 (2008).
Lasonder, E. et al. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature 419, 537–542 (2002).
Florens, L. et al. A proteomic view of the Plasmodium falciparum life cycle. Nature 419, 520–526 (2002).
Sims, P.F. & Hyde, J.E. Proteomics of the human malaria parasite Plasmodium falciparum . Expert Rev. Proteomics 3, 87–95 (2006).
Ribacke, U. Growing Plasmodium falciparum cultures at high parasitemia. in Methods in Malaria Research (eds. Moll, K., Perlmann, H., Scherf, A. & Wahlgren, M.) 8 (American Type Culture Collection, Manassas, Virginia, 2008).
Li, T., Glushakova, S. & Zimmerberg, J. A new method for culturing Plasmodium falciparum shows replication at the highest erythrocyte densities. J. Infect. Dis. 187, 159–162 (2003).
Rivadeneira, E.M., Wasserman, M. & Espinal, C.T. Separation and concentration of schizonts of Plasmodium falciparum by Percoll gradients. J. Protozool. 30, 367–370 (1983).
Trager, W. & Lanners, H.N. Initial extracellular development in vitro of merozoites of Plasmodium falciparum . J. Protozool. 31, 562–567 (1984).
Fernandez, V. Enrichment of late-stage infected erythrocytes in 60% Percoll. in Methods in Malaria Research (eds. Moll, K., Perlmann, H., Scherf, A. & Wahlgren, M.) 25 (American Type Culture Collection, Manassas, Virginia, 2008).
Lelievre, J., Berry, A. & Benoit-Vical, F. An alternative method for Plasmodium culture synchronization. Exp. Parasitol. 109, 195–197 (2005).
Fernandez, V. Separation of Plasmodium falciparum mature stages in Percoll/sorbitol gradients. in Methods in Malaria Research (eds. Moll, K., Perlmann, H., Scherf, A. & Wahlgren, M.) 26–27 (American Type Culture Collection, Manassas, Virginia, 2008).
Ferrer, J. et al. Individual-based model and simulation of Plasmodium falciparum infected erythrocyte in vitro cultures. J. Theor. Biol. 248, 448–459 (2007).
Jensen, J.B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum . Am. J. Trop. Med. Hyg. 27, 1274–1276 (1978).
Jensen, J.B. & Trager, W. Plasmodium falciparum in culture: establishment of additional strains. Am. J. Trop. Med. Hyg. 27, 743–746 (1978).
Ofulla, A.V. et al. Cultivation of Plasmodium falciparum parasites in a serum-free medium. Am. J. Trop. Med. Hyg. 49, 335–340 (1993).
Zolg, J.W., Macleod, A.J., Scaife, J.G. & Beaudoin, R.L. The accumulation of lactic acid and its influence on the growth of Plasmodium falciparum in synchronized cultures. In Vitro 20, 205–215 (1984).
Jensen, M.D., Conley, M. & Helstowski, L.D. Culture of Plasmodium falciparum: the role of pH, glucose, and lactate. J. Parasitol. 69, 1060–1067 (1983).
Lambros, C. & Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65, 418–420 (1979).
Wahlgren, M., Berzins, K., Perlmann, P. & Bjorkman, A. Characterization of the humoral immune response in Plasmodium falciparum malaria. I. Estimation of antibodies to P. falciparum or human erythrocytes by means of microELISA. Clin. Exp. Immunol. 54, 127–134 (1983).
Kutner, S., Breuer, W.V., Ginsburg, H., Aley, S.B. & Cabantchik, Z.I. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: association with parasite development. J. Cell. Physiol. 125, 521–527 (1985).
Krugliak, M. & Ginsburg, H. The evolution of the new permeability pathways in Plasmodium falciparum—infected erythrocytes—a kinetic analysis. Exp. Parasitol. 114, 253–258 (2006).
Krugliak, M., Zhang, J. & Ginsburg, H. Intraerythrocytic Plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol. Biochem. Parasitol. 119, 249–256 (2002).
Moody, A. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15, 66–78 (2002).
World Health Organization. Staining Blood Films with Giemsa Stain (WHO Press, Geneva, Switzerland, 1991).
Goodyer, I.D., Johnson, J., Eisenthal, R. & Hayes, D.J. Purification of mature-stage Plasmodium falciparum by gelatine flotation. Ann. Trop. Med. Parasitol. 88, 209–211 (1994).
Pasvol, G., Weatherall, D.J. & Wilson, R.J. Effects of foetal haemoglobin on susceptibility of red cells to Plasmodium falciparum . Nature 270, 171–173 (1977).
Proudfoot, O., Drew, N., Scholzen, A., Xiang, S. & Plebanski, M. Investigation of a novel approach to scoring Giemsa-stained malaria-infected thin blood films. Malar. J. 7, 62 (2008).
Reilly, H.B., Wang, H., Steuter, J.A., Marx, A.M. & Ferdig, M.T. Quantitative dissection of clone-specific growth rates in cultured malaria parasites. Int. J. Parasitol. 37, 1599–1607 (2007).
Acknowledgements
This work was supported by grants from the Spanish Ministry of Education and Science (BIO2006-12355 and BIO2007-67885) and by the Research Teams Consolidation Programme of the UCM (Research Team 920267 - Comunidad de Madrid). A.R. was on leave from The Pasteur Institute of Iran and holds a fellowship awarded by the AECI-Spanish Ministry of Foreign Affairs. D.M. and C.M. were supported by the Universidad de Cartagena (CO) and the Alban Programme of High Level Scholarships for Latin America, European Union scholarships Nos. E06D101036CO and E07D400516CO. M.L. holds an FPU fellowship from the Spanish Ministry of Education and Science. P. falciparum strains 3D7 and Dd2 were deposited at MR4 by D. Walliker and D.J. Carucci. J. Martinez and E. Albizua from the Haematology Service (University Hospital 12 Octubre, Madrid) kindly provided the patient isolate. Ana Burton read and commented on the manuscript.
Author information
Authors and Affiliations
Contributions
A.R., A.D. and J.M.B. conceived, designed and set up the initial protocol; A.R., D.M., C.M., M.L. and, P.M.-G. cultured the parasites; D.M., C.M., M.L. and P.M.-G. improved the protocol in the laboratory; D.M., C.M., M.L., P.M.-G., A.D. and J.M.B. designed experiments and controls; M.L. grew up the clinical isolate; A.D., A.P. and J.M.B. participated in the analysis and interpretation of the data; all authors contributed to writing different sections of the paper; A.D. and J.M.B. coordinated the laboratory work; J.M.B. wrote the final manuscript versions.
Corresponding author
Rights and permissions
About this article
Cite this article
Radfar, A., Méndez, D., Moneriz, C. et al. Synchronous culture of Plasmodium falciparum at high parasitemia levels. Nat Protoc 4, 1899–1915 (2009). https://doi.org/10.1038/nprot.2009.198
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.198
This article is cited by
-
A PPP-type pseudophosphatase is required for the maintenance of basal complex integrity in Plasmodium falciparum
Nature Communications (2023)
-
The protein aggregation inhibitor YAT2150 has potent antimalarial activity in Plasmodium falciparum in vitro cultures
BMC Biology (2022)
-
Recent metabolomic developments for antimalarial drug discovery
Parasitology Research (2022)
-
Microwave synthesis and antimalarial screening of novel 4-amino benzoic acid (PABA)-substituted pyrimidine derivatives as Plasmodium falciparum dihydrofolate reductase inhibitors
3 Biotech (2022)
-
Molecular docking and antimalarial evaluation of novel N-(4-aminobenzoyl)-l-glutamic acid conjugated 1,3,5-triazine derivatives as Pf-DHFR inhibitors
3 Biotech (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.