Abstract
Opioid transmission and dysregulated prefrontal cortex (PFC) activity have both been implicated in the inhibitory-control deficits associated with addiction and binge-type eating disorders. What remains unknown, however, is whether endogenous opioid transmission within the PFC modulates inhibitory control. Here, we compared intra-PFC opioid manipulations with a monoamine manipulation (d-amphetamine), in two sucrose-reinforced tasks: progressive ratio (PR), which assays the motivational value of an incentive, and differential reinforcement of low response rates (DRLs), a test of inhibitory control. Intra-PFC methylnaloxonium (M-NX, a limited diffusion opioid antagonist) was given to rats in a ‘low-drive’ condition (2-h food deprivation), and also after a motivational shift to a ‘high-drive’ condition (18-h food deprivation). Intra-PFC DAMGO (D-[Ala2,N-MePhe4, Gly-ol]-enkephalin; a μ-opioid agonist) and d-amphetamine were also tested in both tasks, under the low-drive condition. Intra-PFC M-NX nearly eliminated impulsive action in DRL engendered by hunger, at a dose (1 μg) that significantly affected neither hunger-induced PR enhancement nor hyperactivity. At a higher dose (3 μg), M-NX eliminated impulsive action and returned PR breakpoint to low-drive levels. Conversely, intra-PFC DAMGO engendered ‘high-drive-like’ effects: enhancement of PR and impairment of DRL performance. Intra-PFC d-amphetamine failed to produce effects in either task. These results establish that endogenous PFC opioid transmission is both necessary and sufficient for the expression of impulsive action in a high-arousal, high-drive appetitive state, and that PFC-based opioid systems enact functionally unique effects on food impulsivity and motivation relative to PFC-based monoamine systems. Opioid antagonists may represent effective treatments for a range of psychiatric disorders with impulsivity features.
Similar content being viewed by others
INTRODUCTION
Deficient inhibitory control over appetitively motivated behavior occurs in multiple psychiatric disorders; prominent examples include binge-eating disorder and bulimia nervosa, drug addiction, compulsive sexual behavior, and pathological gambling (DSM-5, 2013; Frascella et al, 2010). The neural basis for unregulated appetitive motivation in these disorders is not fully understood. However, considerable evidence implicates functional abnormalities in frontal cortical sites that are engaged by reward-associated cues and that modulate impulsive reward-seeking behavior (Lock et al, 2011; Schienle et al, 2009). Accordingly, human neuroimaging studies have revealed aberrant frontal activity in drug and behavioral addictions, and in eating disorders (Seo et al, 2013; Uher et al, 2004; Volkow et al, 2005). Neuropsychological assessments in individuals with these disorders have shown deficits in frontally mediated processes such as impulse control and decision-making (Lock et al, 2011; Robbins et al, 2012; Schag et al, 2013). At present, the neuropharmacological basis for this frontal dysfunction is unclear. A possible clue derives from the fact that opioid receptor antagonists exhibit some degree of efficacy across several disorders characterized by the loss of control over appetitively motivated behavior (Cambridge et al, 2013; Kim et al, 2001; Mitchell et al, 2007; Volpicelli et al, 1992). This observation could suggest a role for dysregulated opioid signaling, possibly in frontal cortex, in the etiology of inhibitory control deficits. Opioid transmission (particularly in the nucleus accumbens) has been extensively studied in the context of food and drug reward (for example, see Trigo et al, 2010; Zhang et al, 2003). With regard to the modulation of inhibitory control per se, however, opioids have received comparatively little attention. A small number of systemic pharmacology and gene knockout studies have shown that μ-opioid receptor (μOR) signaling promotes impulsivity; however, the brain sites underlying this effect are not fully known (Kieres et al, 2004; Mahoney et al, 2013; Olmstead et al, 2009; Pattij et al, 2009). To date, the possibility that endogenous opioids act within frontal cortex to modulate (or provoke) impulsive reward-seeking action has never been explored.
Here, we studied μOR signaling in the ventromedial prefrontal cortex (vmPFC) across two sucrose-reinforced tasks: differential reinforcement of low response rates (DRLs), which tests the ability to suppress ‘impulsive-like’ responses, and progressive ratio (PR), which probes the motivational value of an incentive. Our aims were: (1) to determine whether endogenous intra-vmPFC opioid signaling is necessary for diminished inhibitory control occurring in a high-drive state, by blocking vmPFC-localized opioid receptors after a motivational shift from 2- to 18-h food restriction; (2) to investigate whether intra-vmPFC μ-opioid receptors (μOR) stimulation is sufficient to cause loss of inhibitory control in a ‘low-drive’ state. Effects on impulsive action in DRL were compared and contrasted with motivational effects in PR. Furthermore, μOR agonist effects were compared with those of intra-vmPFC d-amphetamine (AMPH), to evaluate functional differences between PFC-based opioid vs monoamine systems in the behavioral constructs under study.
MATERIALS AND METHODS
Subjects
Subjects were male Sprague–Dawley rats (Harlan, Madison, WI), weighing 275–300 g upon arrival at the laboratory. Rats were housed in a light- and temperature-controlled vivarium, under a 12 : 12 h light–dark cycle (lights on at 0700 hours). Food and water were available ad libitum, except as indicated for various experiments. Animals were handled daily to reduce stress. Testing occurred between 1200 and 1700 h. All facilities and procedures were in accordance with National Institutes of Health guidelines, and were approved/supervised by the Institutional Animal Care and Use Committee of the University of Wisconsin.
Operant-Behavior Procedures
Operant testing was carried out in standard operant chambers, described in the Supplementary Materials.
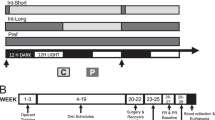
After acclimation to the housing facility, rats underwent an initial training period during which they were maintained at 90±2% of free-feeding body weight using scheduled feedings. During this initial phase, all rats were trained to lever press on a conjoint random-time 30 s/fixed ratio 1 schedule (RT-30 s/FR-1), in which a sucrose pellet was delivered every 30 s regardless of the rats’ behavior while single lever presses also resulted in sucrose pellet delivery. Once the rats were reliably retrieving all pellets during the session (typically within 2–3 days), the RT-30 s component was removed. Hence, at this point, all subjects were responding on an FR-1 schedule.
Next, separate cohorts of rats underwent different training progressions for the PR vs DRL tasks. For PR training, once responding was achieved on FR-1, rats advanced to FR-3, FR-5, and finally PR-2 schedules. The PR-2 contingency consisted of a constant increase in the number of lever presses required to obtain each successive reinforcer (+2 presses, such that one response was required for the first reinforcer, three for the second, five for the third, and so on). PR sessions lasted 120 min. After 2–3 days on the PR-2 schedule, rats were returned to ad libitum food access in their home cages. Thereafter, rats were food deprived for 2 h immediately preceding each testing session. This mild level of food restriction yielded highly stable levels of baseline responding, such that either increases or decreases from baseline (as engendered by experimental manipulations) could be detected. Rats were maintained on the PR-2 schedule until stability was achieved (ie, <10% variability in the number of reinforcers earned in each of three sequential daily testing sessions).
For DRL training, once stability was achieved on FR-1, rats advanced to a variable-interval 15 s schedule (VI-15 s), then to a VI-30 s, and finally to a DRL-15 s schedule. In DRL schedules, after a reinforcer is earned, subjects are required to withhold responding during an unsignaled, fixed time period (in our case, 15 s). Once this delay interval has elapsed, the subject can then respond to earn the next reinforcer. However, each time a ‘premature’ response is emitted (ie, one that is not separated from the previous response by at least 15 s), the delay timer is reset. To achieve optimal performance, therefore, the timing of consecutive responses (inter-response times (IRTs)) must exceed the delay interval. Successful performance in DRL schedules is thought to require intact executive processes of inhibitory control.
After 2–3 days on DRL, rats were switched to ad libitum food access, with 2-h food restriction immediately before each testing session. Rats were then rebaselined under this new food restriction schedule. DRL sessions lasted 20 min.
Experimental Design
Experiment 1: Effects of intra-vmPFC methylnaloxonium (M-NX) on PR and DRL performance
After recovery from surgery, rats were rebaselined on their respective operant tasks (PR-2 or DRL-15 s) under 2-h food restriction, as described above. Upon exhibiting stable baseline responding (no more than±10% variability over three consecutive testing days), rats were acclimated to the microinfusion procedures with saline injections. Rats were rebaselined after these injections, whereupon drug testing commenced.
Food was removed from the home cages 18 h before each testing day, resulting in a motivational shift from their ‘low-drive’ baseline state into a ‘high-drive’ state. On testing days, rats received intra-vmPFC infusions (0, 1, or 3 μg/0.5 μl M-NX) and were placed into the operant chambers for their respective PR or DRL session. Separate groups of rats were used for the two operant tasks (N=8 for PR; N=7 for DRL). To ensure that peak drug effects would coincide with the 20 min DRL sessions, infusions were given 15 min before testing, with rats placed in their home cages without food for the 15-min postinjection period. PR sessions, however, were longer (120 min); thus, to minimize the chance that the sessions would outlast the duration of drug effects, rats were placed into operant chambers for PR testing immediately after infusions. Doses were counterbalanced according to Latin square designs, with two interim days of drug-free testing (under 2-h food deprivation) separating the drug-infusion days. Two days after completion of all doses, rats underwent three additional testing days on which they received intra-vmPFC M-NX infusions (0, 1, or 3 μg/0.5 μl) in the baseline 2-h food deprivation state. Again, dose order was counterbalanced across subjects according to a Latin square design. One drug-free interim testing day separated each drug-infusion day.
After completion of this second M-NX dose–response assessment, rats were tested with intra-PFC saline or M-NX (1 μg), under 18-h food deprivation, in a behavioral-observation procedure. The PR and DRL groups were split, with one part of each group receiving intra-PFC M-NX (1 μg; N=8; 4 PR and 4 DRL rats), and the other part receiving intra-PFC saline (N=7; 3 PR and 4 DRL rats). Procedural details of the behavioral-observation test are given below.
Experiment 2: Effects of intra-vmPFC DAMGO and AMPH on PR and DRL responding
Postsurgery baselining, and preliminary sham and saline infusions, were given as described in Experiment 1. For PR, intra-vmPFC DAMGO (D-[Ala2,N-MePhe4, Gly-ol]-enkephalin; 0, 0.25, and 2.5 μg/0.5 μl) and intra-vmPFC AMPH (0, 0.75, 1.5, and 5.0 μg/0.5 μl) were tested in separate groups of rats (N=6 for DAMGO; N=6 for AMPH). For each drug, doses were given according to within-subjects Latin square designs, with 2–3 interim days of drug-free baseline testing separating the drug-infusion days. For DRL, DAMGO (0, 0.25, and 2.5 μg/0.5 μl) and AMPH (0, 1.5, and 5.0 μg/μl) were tested in the same rats (N=8), in counterbalanced order (ie, half the rats received DAMGO first, the other half, AMPH first). All testing with DAMGO and AMPH was carried out under 2-h food deprivation.
Behavioral-Observation Procedure
Rats were habituated to clear polycarbonate cages (9.5 in width × 17 in length × 8 in height), identical to the home cages except for wire grid floors. Sucrose pellets were placed in glass jars affixed to the testing cage floors; water was available in overhead water bottles. Thirty-minute habituation sessions were carried out on two sequential days. On the third day, rats were food deprived for 18 h, and rats were injected with their respective treatments, whereupon they were placed in the testing cages and videotaped with a digital camcorder for 75-min sessions. For the first 30 min of each session, a wire covering was placed over the sucrose jars so that the sucrose could be seen and smelled, but not accessed. The wire covering was then removed, and rats were allowed free access to the sucrose (and water) for 45 min. An experimenter blind to treatment viewed the digital files. Spontaneous ambulation, rearing, drinking bouts, and grooming bouts were recorded both pre- and postscreen removal. In addition, screen approaches were recorded before screen removal, and sucrose-eating bouts recorded after screen removal. Behaviors were recorded using an event recorder interfaced to a PC-based laptop computer (Bakshi and Kelley, 1991).
Surgical Procedures
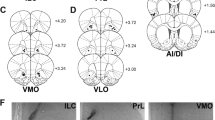
Stereotaxic surgery under isoflurane anesthesia was carried out as described elsewhere (Perry et al, 2009). Bilateral guide cannulae were aimed at the vmPFC (near the dorsal border of infralimbic cortex). We have found in previous studies that strong μ-opioid-driven feeding responses can be elicited from this area (Mena et al, 2011). Coordinates for cannulae placements were as follows: anteroposterior, +3.0 mm anterior to bregma; mediolateral, ±2.2 mm from the midline; dorsoventral, −2.7 mm from the skull surface (2.5 mm above the final infusion site). Additional procedural details are provided in the Supplementary Materials.
Microinfusion Procedures and Drugs
Intracerebral microinfusions were carried out according to standard procedures (see Perry et al., 2009). Details are provided in the Supplementary Materials. M-NX (a lipophobic derivative of the opioid receptor antagonist, naloxone), DAMGO (μ-opioid agonist), and the nonspecific monoamine releaser AMPH were obtained from Sigma-Aldrich (St Louis, MO). The intra-PFC AMPH dose range used here is similar to that used in prior studies (Vezina et al, 1991; Yates et al, 2014), and is clearly behaviorally active when infused into the nucleus accumbens (Bakshi and Kelley, 1991; Kelley and Delfs, 1991). All drugs were dissolved in sterile 0.9% saline immediately before infusions.
Statistical Analyses
Data were analyzed using within-subjects factorial ANOVAs as required by the experimental designs. Contingent upon significance in the ANOVAs, post hoc comparisons among means were conducted with Tukey’s test. Data from the behavioral-observation experiment were analyzed with unpaired t-tests. The level of statistical significance was set at P<0.05 for all experiments.
RESULTS
Blockade of vmPFC Opioid Receptors with M-NX Reversed the Impulsivity Observed in a High-Drive State
At the highest dose of M-NX (3 μg), and only at this dose, three of the seven rats failed to respond in the DRL sessions. These rats did not emit any lever presses during the 20-min sessions. However, inspection of their behavior in the operant chambers revealed no apparent behavioral impairment. Locomotion, rearing, sniffing, and so on inside the chambers was indistinguishable from their behavior on other test days, and was also indistinguishable from rats in their cohort that successfully responded under 3 μg M-NX. Furthermore, there was no systematic difference in injector placement for any of the rats within this M-NX experiment. Hence, to ensure the veracity of statistical inferences, the data were analyzed in two ways: first, with all doses included, omitting rats that did not respond at 3 μg M-NX; second, with all rats included, omitting the 3-μg dose. Both analyses support the same conclusions, and both are presented here.
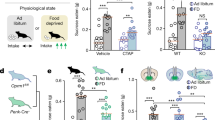
In DRL, shifting rats from a low-drive state (2-h food deprivation) to a high-drive state (18-h food deprivation) significantly impaired response efficiency (main effect of drive: F(1, 3)=75.54, P=0.003 with all doses; F(1,6)=41.24, P=0.0007 with all rats). Efficiency ratios were calculated by dividing the number of reinforced lever presses by the total number of presses (ie, reinforced+unreinforced) for each session, and expressing these ratios as percentages. Intra-vmPFC M-NX significantly reversed this hunger-induced efficiency decrement (dose × drive interaction: F(2,6)=6.69, P=0.029 with all doses; F(1,6)=7.36, P=0.035 with all rats; Figure 1a). In contrast, M-NX had no effect on nose-poking into the food hopper during the DRL sessions. Shifting rats to the high-drive state significantly elevated nose-poking (main effect of drive: F(1,3)=18.84, P=0.023 for all doses; F(1,6)=8.40, P=0.027 for all rats). Intra-vmPFC M-NX did not, however, alter nose-poking under either the low-drive state, nor did it alter the elevated rate of nose-poking observed in the high-drive state (Fs=0.24–3.18, not significant (NS); Figure 1b).
Treatment with intra-ventromedial prefrontal cortex (vmPFC) methylnaloxonium (M-NX) reversed impulsive responding in the differential reinforcement of low rate (DRL) task after rats were shifted from 2- to 18-h food deprivation states. Rats (n=7) shifted from a low-drive (2 h) to high-drive (18 h) state showed impaired response efficiency (ie, the ratio of reinforced lever presses to total lever presses (a) and increased nose-poking behavior (b). Intra-vmPFC M-NX attenuated deficits in response efficiency but had no effect on nose-poking behavior. In (a), *P<0.05, main effect of drive; #P<0.05, different from all low-drive means and from high-drive+3-μg M-NX. In (b), *P< 0.05, main effect of drive. Spontaneous locomotor activity (‘movements’, the sum of horizontal movement (locomotion—‘loco’) and vertical movement (rearing—‘rear’) was not effected by M-NX (c). Analysis of inter-response times (IRTs; timing of consecutive responses) (d) revealed that the motivational shift to a high-drive state resulted in an increase in responding across the four shortest ‘inefficient-response’ IRT bins; inhibitory control was significantly rescued by intra-vmPFC M-NX (1 μg). In (d), *P<0.05, different from all ‘low-drive’ means; #P<0.05, different from ‘high-drive’+saline, in each respective time bin. Error bars depict 1 SEM.
Further analysis was conducted on the temporal spacing of lever presses, comparing vehicle and the 1-μg M-NX dose (ie, the dose at which all rats responded). Responses for each session were grouped according to their IRTs; frequency distributions of responses by IRT bin were generated. For clarity, IRTs were collapsed into 3-s bins. Inefficient lever presses consisted of unreinforced, ‘premature’ responses that did not meet the 15-s IRT requirement. Reinforced lever presses, on the other hand, were spaced at least 15-s apart. Rapid, closely spaced responses in the ‘ultra-short’ IRT bin (0–3 s) are thought to reflect loss of inhibitory control (Doughty and Richards, 2002). Intra-vmPFC M-NX significantly reversed the hunger-induced augmentation of inefficient lever pressing (drug × drive × IRT bin: F(5,30)=3.13, P=0.022; Figure 1d). Following this three-way interaction, data were further analyzed by time bin using ANOVAs, followed by Tukey’s tests. For the first four ‘inefficient IRT’ bins, saline-treated rats in the high-drive state emitted significantly more inefficient responses than they did in the low-drive state; in these time bins, M-NX treatment returned high-drive responding to low-drive levels (Figure 1d; Fs=5.4–10.0; Ps=0.008–0.0004). Importantly, intra-vmPFC M-NX failed to decrease the number of reinforced responses under either drive condition. Finally, M-NX did not alter responding, inefficient or otherwise, when given in the low-drive condition.
Intra-vmPFC Opioid Receptor Blockade Attenuated Food Motivation in the PR Task
Shifting rats from 2- to 18-h food deprivation markedly increased responding in the PR task, reflected as both increased responding on the active lever and in increased ‘breakpoint’ (ie, the last completed ratio, calculated by applying the formula X+(X−1), where X is the number of cumulative reinforcers for each session) (main effects of drive: F(1,7)=32.08, P=0.0008 for active lever presses; F(1,7)=49.35, P=0.002 for breakpoint; Figures 2a and c). Eighteen hours of food deprivation also increased responding on the inactive lever (F(1,7)=8.45, P=0.023) and augmented nose-poking (F(1,7)=12.96, P=0.009; Figures 2b and d).
Treatment with intra-ventromedial prefrontal cortex (vmPFC) methylnaloxonium (M-NX) attenuated the hunger-induced amplification of food motivation in the progressive ratio task. Rats (n=7) shifted from a low-drive (2 h) to high-drive (18 h) state showed increases in responding on the active (a) and inactive (b) levers. The motivational shift also resulted in a higher ‘breakpoint’ (ie, the last completed ratio, c). Intra-vmPFC infusions of M-NX (3 μg) restored active lever presses and breakpoint to ‘low-drive’ levels. Increases in nose-poking into the food hopper (d) were not altered by M-NX administration. Progressive ratio sessions were 2 h long. *P<0.05; main effect of drive; #P<0.05, different from high drive+saline; †P<0.05, different from ‘high-drive+1 μg M-NX’. Error bars depict 1 SEM.
Intra-vmPFC M-NX dose-dependently reduced active lever presses (main effect of drug: F(2,14)=6.81, P=0.0086) and breakpoint (F(2,14)=9.98, P=0.002). These drug effects were due mainly to actions in the high-drive condition (drug × drive interactions; F(2,14)=5.66, P=0.016 for active lever presses; F(2,14)=5.11, P=0.022 for breakpoint; Figure 2). Post hoc means comparisons indicated that the high M-NX dose (3 μg), given in the 18-h deprivation state, returned active lever presses and breakpoint from ‘high-drive’ to ‘low-drive levels’. Nevertheless, this dose did not alter responding when given in the low-drive state. Importantly, the lower dose (1 μg) had no statistically significant effects in PR in either the high- or low-drive state. Finally, intra-vmPFC M-NX altered neither inactive lever responding nor nose-poking into the food hopper.
Intra-vmPFC Opioid Receptor Blockade did not Impair Spontaneous Activity, Sucrose-Directed Approach, or Sucrose Intake in 18-h Food-Deprived Rats
To further assess whether the apparent ‘rescue’ of inhibitory control by 1 μg M-NX was the nonspecific consequence of general motoric or motivational impairments, rats from the M-NX DRL and PR experiments were challenged with either saline or 1 μg M-NX, and their spontaneous activity, feeding, and food-approach behavior was assessed in a behavioral-observation procedure (see Materials and Methods for full details). Briefly, in the first 30 min of this test, behaviors were recorded and rats had free access to water, but a see-through wire screen prevented access to sucrose pellets (the same pellets as were used in the operant chambers). Next, the screen was removed, and rats were permitted access to sucrose pellets and water for 45 min. There were no significant effects of M-NX on ambulation, rearing, grooming, or drinking in either the pre- or postscreen phase, nor did intra-vmPFC M-NX alter screen approaches or postscreen sucrose intake (t-values=−1.3 to 2.1, NS). Activity with the screen in place, summarized as horizontal+vertical movement (ie, ambulation counts+rearing counts), is shown in Figure 1c. All additional measures from this study are summarized in the Supplementary Materials and Supplementary Table S1. These observations indicate that blockade of vmPFC-localized opioid receptors with 1 μg M-NX does not impair spontaneous motor activity, food approach, or food intake (when food is available with low effort), arguing against the presence of gross motor or motivational impairments at this dose.
Intra-vmPFC DAMGO, but not AMPH, Augmented Food Motivation and Impaired Inhibitory Control of Food-Seeking Responses
In the PR task, intra-vmPFC DAMGO given under the low-drive state increased active lever pressing (F(2,10)=10.41, P=0.004), breakpoint (F(2,10)=21.42, P=0.0002), and nose-poking (F(2,10)=21.24, P=0.0003), but did not alter inactive lever pressing. These effects are summarized in Figure 3 and Supplementary Figure S1. Intra-vmPFC AMPH was, however, devoid of effects on any of these measures (Fs=0.67–2.58, NS; Figure 3b, d, and f). Two days after completion of AMPH dose–response testing, the same rats were challenged with intra-vmPFC DAMGO (2.5 μg) as a positive control. DAMGO produced a significant response, relative to saline and AMPH, on breakpoint (F(4,20)=6.67, P=0.0014), active lever pressing (F(4,20)=5.75, P=0.003), and nose-poking (F(4,20)=18.50, P<0.0001), but not inactive lever pressing (F(4,20)=1.36, NS).
Rats (n=6) treated with intra-ventromedial prefrontal cortex (vmPFC) infusions of the μ-opioid agonist DAMGO (D-[Ala2,N-MePhe4, Gly-ol]-enkephalin) during the low-drive state showed increases in food motivation, whereas rats (n=6) treated with intra-vmPFC infusions of d-amphetamine (AMPH) did not exhibit any change in pressing for sucrose reward in the progressive ratio task. Intra-vmPFC DAMGO increased responding on the active lever (a), resulting in an increased breakpoint (ie, the last completed ratio, c). Nose-poking was also increased (e). Intra-vmPFC AMPH infusions had no effect on active lever pressing (b), breakpoint (d), or nose-poke behavior (f). The same rats, when challenged with DAMGO, showed significant increases in all three measures. In (a), (c), and (e), *P<0.05, different from saline; #P<0.05, difference between the two DAMGO doses. In (b), (d), and (f), *P<0.05, different from all within-subject saline and AMPH treatments. Error bars depict 1 SEM.
Intra-vmPFC DAMGO also produced ‘high-drive-like’ effects in the DRL task. Because a subset of rats (4 out of 12) did not respond in DRL at the highest DAMGO dose (2.5 μg), the data were analyzed in two ways: with all doses included, omitting rats that did not respond at 2.5 μg DAMGO; and with all rats included, omitting the 2.5-μg dose. Both analyses are presented here. Note also that, in this experiment, DAMGO and AMPH were tested in the same rats in counterbalanced order. There were no effects of drug order for any of the DRL measures (Fs=0.06–0.32, NS).
DAMGO, but not AMPH, robustly diminished task efficiency (F(4,28)=11.12, P<0.0001 with all doses; F(3,33)=23.88, P<0.0001 with all rats). Post hoc analyses indicated that, at the 0.25-μg DAMGO dose, efficiency scores were significantly lower than those seen with saline or either of the two AMPH doses, and at the 2.5-μg DMGO dose, efficiency scores were lower than for 5-μg AMPH. Furthermore, efficiency levels at both AMPH doses were virtually identical to saline (see Figure 4a). DAMGO also significantly elevated nose-poking into the food hopper, an effect similar to that seen with 18-h food deprivation (see previous section) (F(4,28)=15.07, P<0.0001 with all doses; F(3,33)=17.31, P<0.0001 with all rats; see Figure 4b). IRT analysis, focusing on comparisons among saline, 0.25 μg DAMGO (the dose at which all rats responded), and the highest AMPH dose (5.0 μg), indicated that DAMGO significantly altered responding relative to saline or AMPH (IRT bin × drug interaction: F(10, 110)=13.89, P<0.0001). Post hoc means comparisons revealed that, for all ‘inefficient-response’ bins (ie, IRTs <15 s), the number of lever presses was significantly greater for DAMGO-treated relative to saline- or AMPH-treated rats (see Figure 4c). Numbers of reinforced responses, however, did not differ across the treatment groups.
Rats (n=8) treated with intra-ventromedial prefrontal cortex (vmPFC) infusions of DAMGO (D-[Ala2,N-MePhe4, Gly-ol]-enkephalin) displayed increased impulsivity in the differential reinforcement of low response rate (DRL) task. In the same rats, d-amphetamine (AMPH) failed to alter DRL responding. Intra-vmPFC DAMGO infusions impaired response efficiency (a) and increased nose-poking behavior (b). Inter-response times (IRT) analysis revealed that DAMGO (0.25 μg) increased responding for all ‘inefficient-response’ IRT bins (c). The number of reinforced responses (ie, the >15-s bin) did not differ between groups. In (a), *P<0.05, different from saline; #P< 0.05, different from both AMPH doses; †P<0.05, different from AMPH-5 μg. In (b), *P<0.05, different from saline; #P<0.05, different from both AMPH doses. In (c), *P<0.05, different from saline; #P<0.05, different from AMPH in each respective time bin. Error bars depict 1 SEM.
Analysis of Intra-vmPFC Injector Placements
As shown in Figure 5, placements fell mainly in the infralimbic area of medial PFC, with some placements in the ventral prelimbic territory. For the DAMGO/AMPH DRL study, it was noted that the rostrocaudal range of placements was greater compared with other experiments. Therefore, we analyzed efficiency, nosepokes, reinforced responses, and inefficient responses in the four rats with the most rostral placements, and the four with the most caudal placements, with ‘placement’ as a between-subjects factor in the ANOVA. This analysis failed to reveal drug × placement interactions for any of the aforementioned measures (Fs=0.19–1.18, NS), indicating that drug effects did not differ across the rostrocaudal placements in this experiment.
Chartings for injector placements in all experiments (a). Different shapes (for different experiments) depict the placement of injector tips. Photomicrographs in (b) illustrate representative examples for the DAMGO (D-[Ala2,N-MePhe4, Gly-ol]-enkephalin) progressive ratio (PR), DAMGO and d-amphetamine (AMPH) DRL, and methylnaloxonium (M-NX) differential reinforcement of low response rate (DRL) experiments.
DISCUSSION
The present findings reveal a novel role of vmPFC-based opioid receptor signaling in the control of food-related motivation and impulsivity. Blockade of intra-vmPFC opioid receptors with M-NX almost completely reversed the deficit in DRL task efficiency incurred by shifting rats from a low-drive (2-h food deprivation) to a high-drive (18-h food deprivation) state. This inhibitory-control improvement did not appear to be an artifact of drug-induced motor slowing or motivational impairment, for several reasons. First, neither reinforced DRL responses, nose-poking into the food hopper, nor general exploratory activity were affected by intra-vmPFC M-NX. Second, the 1-μg M-NX dose, which strongly reduced hunger-induced inefficient responding in DRL, failed to significantly alter PR breakpoint, inactive-lever responses, or nose-poking. At a slightly higher dose (3 μg), intra-vmPFC M-NX attenuated the hunger-induced amplification of PR breakpoint; again, there were no effects on inactive-lever responses and nose-poking. Third, M-NX produced no behavioral effects at any dose in the baseline, low-drive state. Conversely, stimulation of vmPFC-localized μORs in the low-drive state recapitulated behavioral features of 18-h deprivation: notably, amplification of PR breakpoint and decrease in DRL efficiency. Nevertheless, DAMGO did not provoke inactive-lever pressing, suggesting that the behavioral changes were not the outcome of nonspecific motoric arousal. It is important to note that M-NX is a nonspecific opioid antagonist whose utility arises from its limited tissue diffusion, allowing for better localization of action. Future studies using more specific μ-specific antagonists would establish whether the ‘rescue’ of impulse control in hunger is reliant specifically upon blockade of the μOR subtype. Nevertheless, the present findings represent the first demonstration (to our knowledge) that intra-vmPFC opioid signaling is both necessary and sufficient for the expression of inhibitory-control deficits in the context of food-seeking behavior.
Inefficient responding, including ‘bursts’ of closely spaced responses (ie, those characterized by ultrashort IRTs), is a standard feature of DRL response topography and has been argued to represent an ‘impulsivity-like’ failure to suppress prepotent but disadvantageous action (Doughty and Richards, 2002; Sokolowski and Salamone, 1994). Strikingly, the lower dose of intra-vmPFC M-NX ‘rescued’ DRL response efficiency in the high-drive state without significantly altering the general activational properties of this state (nose-poking, hyperactivity, breakpoint enhancement). This suggests a possible dose dissociation between prefrontal processes governing inhibitory control mechanisms and recruiting motivational mechanisms—that is, impulsive action at the 1-μg dose was reduced, even though ‘wanting’ of the goal was relatively intact. This inference is further supported by the fact that 1-μg M-NX did not alter sucrose approach or intake in the behavioral-observation test. Future studies using more demanding PR and DRL schedules are warranted to further test this interesting possibility. The fact that M-NX was devoid of effects in the 2-h deprivation state suggests that basal vmPFC opioid tone is low, but is elevated (thereby becoming behaviorally relevant) in a state of heightened arousal/appetitive drive. Amplification of vmPFC μOR signaling with exogenous DAMGO administration both impaired DRL performance and increased PR breakpoint. Taken together, these results demonstrate a role for state-related opioid signaling in modulating the inter-related processes of appetitive motivation and inhibitory control over food-seeking behavior.
The present results add to a growing body of evidence that μ-opioids mediate functionally unique effects relative to other PFC-based neurochemical systems. The striking dissociation between DAMGO and AMPH shown here agrees with our previous finding that, whereas intra-vmPFC DAMGO provoked hyperphagia, a wide variety of intra-vmPFC dopaminergic, noradrenergic, or serotonergic agonists or antagonists failed to do so (Mena et al, 2011). Indeed, to our knowledge, no other neurochemical manipulation of the PFC recapitulates the entire μ-opioid ‘behavioral phenotype’ of food intake, food-reinforced operant responding, and impulsivity. Moreover, the present results highlight important differences in the behavioral actions of AMPH in the PFC vs nucleus accumbens (Acb). The dose range of AMPH used here engenders significant hyperactivity and robustly increases responding in PR and other operant tasks when infused into the Acb (Bakshi and Kelley, 1991; Kelley and Delfs, 1991; Zhang et al, 2003), in clear contrast to the lack of effects seen in the present study. With regard to impulsive action, systemic infusion of AMPH provokes premature responding in the five-choice serial reaction time task; this effect is blocked by intra-Acb dopamine antagonist infusions, naloxone infusions, or 6-hydroxydopamine lesions (Cole and Robbins, 1989; Pattij et al, 2007; Wiskerke et al, 2011). Furthermore, intra-Acb AMPH infusion strongly elevates inefficient DRL responding (Neill, 1976). These results contrast the lack of intra-vmPFC AMPH effects in either PR or DRL seen here, or in a prior study reporting negative effects of intra-PFC AMPH (in a similar dose range as used here) on a delay-discounting procedure (Yates et al, 2014). In fact, prior work has shown that 6-OHDA lesions of the PFC cause inefficient responding in DRL (Sokolowski and Salamone, 1994), and blockade of PFC-localized D1 and D2 dopamine receptors engenders impulsive choice in a delayed-reinforcement task (Pardey et al, 2013). Conversely, intra-PFC AMPH attenuated the hyperactivity induced by intra-Acb AMPH (Vezina et al, 1991). Taken together, these results indicate that an optimal level of PFC-based dopamine transmission is required for intact inhibitory control. Pharmacologically elevating PFC monoamine release with AMPH does not improve inhibitory control in the baseline state (as seen here), but may become relevant when there is a challenge to the system. This pattern of results could reflect a ‘stabilizing’ action of monoamines on cortical networks, which is not apparent when network efficiency is already near its ceiling. Furthermore, the fact that intra-PFC opioid agonists produce the same effects on inhibitory control as do intra-PFC 6-OHDA lesions or dopamine antagonist infusions could indicate oppositional effects of PFC-based opioid and dopamine systems. It is interesting to posit that optimal levels of dopamine could ‘buffer’ against the disruptive effects of opioid signaling in heightened arousal/drive states. It would be interesting, for example, to assess whether intra-vmPFC AMPH improves DRL performance in a high-drive state.
Presently, the mechanisms by which opioids modulate the PFC cellular network are unclear. The few studies that have been carried out, however, indicate that μOR signaling profoundly modulates cortical activity. Endogenous opioid peptides (present in the PFC as enkephalin (ENK), β-endorphin, and endomorphins; Ferezou et al (2007) and Martin-Schild et al (1999)) act upon μORs at key points within the PFC cellular network. PFC μORs are localized on GABA interneurons (not pyramidal cells) (Ferezou et al, 2007; Taki et al, 2000), and endogenous ENK acts at these receptors to suppress interneuron activity and to reduce inhibitory currents onto pyramidal cells (Ferezou et al, 2007; Witkowski and Szulczyk, 2006). This action removes an inhibitory component from cellular network function, presumably disinhibiting the network in a manner similar to μ-opioid actions in the hippocampus (McQuiston and Saggau, 2003). μORs also appear to function as heteroreceptors on thalamocortical nerve terminals, interacting with serotonin 2A receptors to modulate glutamate release (Marek and Aghajanian, 1998; Marek et al, 2001). These multiple actions have the potential to strongly shape patterns of activation in the PFC, altering ongoing discharge patterns, changing input/output mappings, and enacting other processes that govern PFC engagement of subcortical systems. Our recent work, for example, suggests that intra-vmPFC μOR stimulation engenders heightened glutamate signaling in multiple terminal fields, including the Acb and hypothalamus (Mena et al, 2013). Considering evidence of PFC–Acb functional connections in modulating drug reinstatement, attentional performance, and other processes that tax inhibitory control (Bossert et al, 2012; Christakou et al, 2004; Peters et al, 2008), this pathway may be particularly relevant for PFC-opioid-induced impulsive action seen here.
Human-imaging studies suggest that exaggerated activity within select frontal sites, including ventromedial aspects of PFC and anterior cingulate cortex (ACC) (areas roughly homologous to the site studied here), contributes to inhibitory-control deficits in a variety of psychiatric disorders characterized by dysregulated appetitive motivation (Karhunen et al, 2000; Schienle et al, 2009; Seo et al, 2013; Uher et al, 2004). The present results join a growing number of studies, indicating that these cortical sites represent crucial loci of clinically relevant opioid action. In humans, ligand PET studies have demonstrated frontal cortical μ-opioid peptide release in association with sweetened-alcohol drinking (Mitchell et al, 2012) and μOR upregulation in frontal sites including the PFC and ACC robustly predicts the severity of craving and rapidity of relapse in cocaine users (Gorelick et al, 2008; Zubieta et al, 1996). μORs are upregulated in the PFC (along with the Acb and amygdala) in individuals with trait impulsivity, and these individuals display exaggerated stressor-induced PFC opioid release (Love et al, 2009). In animal studies, PFC-localized μ-opioid peptides are elevated after exposure to ‘binge-like’ palatable feeding or cocaine self-administration schedules, and in rats predisposed to excessive ethanol intake (Blasio et al, 2013; Morganstern et al, 2012). Finally, intra-PFC naloxone infusion reduced food-reinforced PR responding in rats that had experienced a ‘binge’-inducing schedule of sugar access (Blasio et al, 2013). Taken together with the present results, these studies raise the possibility that supernormal opioid transmission could underlie the frontal cortical dysregulation observed in fMRI studies across multiple binge-type disorders. The PFC may therefore represent a crucial site at which naltrexone and similar drugs act to ameliorate a bingeing endophenotype.
An important future goal is to determine whether endogenous PFC μ-opioid signaling has a role in mediating executive deficits in other types of high-arousal states, beyond food-motivated states. If so, opioid-blocking drugs may have clinical utility beyond current use in binge-type eating disorders and alcoholism; for example, these drugs may also improve performance in a wider range of psychiatric conditions in which extreme arousal impedes executive function. The present findings provide a mechanistic justification for pursuing such possibilities.
Funding and Disclosure
The authors declare no conflict of interest.
References
Bakshi VP, Kelley AE (1991). Dopaminergic regulation of feeding-behavior. 2. Differential-effects of amphetamine microinfusion into 3 striatal subregions. Psychobiology 19: 233–242.
Blasio A, Steardo L, Sabino V, Cottone P (2013). Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict Biol 19: 652–662.
Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M et al (2012). Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci 32: 4982–4991.
Cambridge VC, Ziauddeen H, Nathan PJ, Subramaniam N, Dodds C, Chamberlain SR et al (2013). Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people. Biol Psychiatry 73: 887–894.
Christakou A, Robbins TW, Everitt BJ (2004). Prefrontal cortical–ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci 24: 773–780.
Cole BJ, Robbins TW (1989). Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res 33: 165–179.
Doughty AH, Richards JB (2002). Effects of reinforcer magnitude on responding under differential-reinforcement-of-low-rate schedules of rats and pigeons. J Exp Anal Behav 78: 17–30.
DSM-5 (2013) Diagnostic and Statistical Manual of Mental Disorders: DSM-55th edn. American Psychiatric Association: Washington, DC. p. xliv, 947pp.
Ferezou I, Hill EL, Cauli B, Gibelin N, Kaneko T, Rossier J et al (2007). Extensive overlap of mu-opioid and nicotinic sensitivity in cortical interneurons. Cerebral cortex (New York, NY: 1991) 17: 1948–1957.
Frascella J, Potenza MN, Brown LL, Childress AR (2010). Shared brain vulnerabilities open the way for nonsubstance addictions: carving addiction at a new joint? Ann N Y Acad Sci 1187: 294–315.
Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino ML et al (2008). Brain mu-opioid receptor binding: relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology 200: 475–486.
Karhunen LJ, Vanninen EJ, Kuikka JT, Lappalainen RI, Tiihonen J, Uusitupa MI (2000). Regional cerebral blood flow during exposure to food in obese binge eating women. Psychiatry Res 99: 29–42.
Kelley AE, Delfs JM (1991). Dopamine and conditioned reinforcement. I. Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology 103: 187–196.
Kieres AK, Hausknecht KA, Farrar AM, Acheson A, de Wit H, Richards JB (2004). Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology 173: 167–174.
Kim SW, Grant JE, Adson DE, Shin YC (2001). Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry 49: 914–921.
Lock J, Garrett A, Beenhakker J, Reiss AL (2011). Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am J Psychiatry 168: 55–64.
Love TM, Stohler CS, Zubieta JK (2009). Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry 66: 1124–1134.
Mahoney MK, Silveira MM, Olmstead MC (2013). Increased impulsive action in rats: effects of morphine in a short and long fixed-delay response inhibition task. Psychopharmacology 230: 569–577.
Marek GJ, Aghajanian GK (1998). 5-Hydroxytryptamine-induced excitatory postsynaptic currents in neocortical layer V pyramidal cells: suppression by mu-opiate receptor activation. Neuroscience 86: 485–497.
Marek GJ, Wright RA, Gewirtz JC, Schoepp DD (2001). A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience 105: 379–392.
Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE (1999). Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Compar Neurol 405: 450–471.
McQuiston AR, Saggau P (2003). Mu-opioid receptors facilitate the propagation of excitatory activity in rat hippocampal area CA1 by disinhibition of all anatomical layers. J Neurophysiol 90: 1936–1948.
Mena JD, Sadeghian K, Baldo BA (2011). Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci 31: 3249–3260.
Mena JD, Selleck RA, Baldo BA (2013). Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci 33: 18540–18552.
Mitchell JM, O'Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL (2012). Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Science Transl Med 4: 116ra6.
Mitchell JM, Tavares VC, Fields HL, D'Esposito M, Boettiger CA (2007). Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology 32: 439–449.
Morganstern I, Liang S, Ye Z, Karatayev O, Leibowitz SF (2012). Disturbances in behavior and cortical enkephalin gene expression during the anticipation of ethanol in rats characterized as high drinkers. Alcohol 46: 559–568.
Neill DB (1976). Frontal-striatal control of behavioral inhibition in the rat. Brain Res 105: 89–103.
Olmstead MC, Ouagazzal AM, Kieffer BL (2009). Mu and delta opioid receptors oppositely regulate motor impulsivity in the signaled nose poke task. PLoS One 4: e4410.
Pardey MC, Kumar NN, Goodchild AK, Cornish JL (2013). Catecholamine receptors differentially mediate impulsive choice in the medial prefrontal and orbitofrontal cortex. J Psychopharmacol 27: 203–212.
Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM (2007). Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology 191: 587–598.
Pattij T, Schetters D, Janssen MC, Wiskerke J, Schoffelmeer AN (2009). Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology 205: 489–502.
Perry ML, Baldo BA, Andrzejewski ME, Kelley AE (2009). Muscarinic receptor antagonism causes a functional alteration in nucleus accumbens mu-opiate-mediated feeding behavior. Behav Brain Res 197: 225–229.
Peters J, LaLumiere RT, Kalivas PW (2008). Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28: 6046–6053.
Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD (2012). Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci 16: 81–91.
Schag K, Teufel M, Junne F, Preissl H, Hautzinger M, Zipfel S et al (2013). Impulsivity in binge eating disorder: food cues elicit increased reward responses and disinhibition. PLoS One 8: e76542.
Schienle A, Schafer A, Hermann A, Vaitl D (2009). Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry 65: 654–661.
Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R (2013). Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry 70: 727–739.
Sokolowski JD, Salamone JD (1994). Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res 642: 20–28.
Taki K, Kaneko T, Mizuno N (2000). A group of cortical interneurons expressing mu-opioid receptor-like immunoreactivity: a double immunofluorescence study in the rat cerebral cortex. Neuroscience 98: 221–231.
Trigo JM, Martin-Garcia E, Berrendero F, Robledo P, Maldonado R (2010). The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend 108: 183–194.
Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW et al (2004). Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry 161: 1238–1246.
Vezina P, Blanc G, Glowinski J, Tassin JP (1991). Opposed behavioural outputs of increased dopamine transmission in prefrontocortical and subcortical areas: a role for the cortical D-1 dopamine receptor. Eur J Neurosci 3: 1001–1007.
Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS et al (2005). Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neuroscience 25: 3932–3939.
Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP (1992). Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49: 876–880.
Wiskerke J, Schetters D, van Es IE, van Mourik Y, den Hollander BR, Schoffelmeer AN et al (2011). mu-Opioid receptors in the nucleus accumbens shell region mediate the effects of amphetamine on inhibitory control but not impulsive choice. J Neurosci 31: 262–272.
Witkowski G, Szulczyk P (2006). Opioid mu receptor activation inhibits sodium currents in prefrontal cortical neurons via a protein kinase A- and C-dependent mechanism. Brain Res 1094: 92–106.
Yates JR, Perry JL, Meyer AC, Gipson CD, Charnigo R, Bardo MT (2014). Role of medial prefrontal and orbitofrontal monoamine transporters and receptors in performance in an adjusting delay discounting procedure. Brain Res 1574: 26–36.
Zhang M, Balmadrid C, Kelley AE (2003). Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci 117: 202–211.
Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ (1996). Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med 2: 1225–1229.
Acknowledgements
This work was funded by National Institute of Mental Health R01 Grant MH074723, a University of Wisconsin-Madison Vilas Life Cycle Award, and research funds from the Graduate School of the University of Wisconsin-Madison (PI on these awards: Dr Brian A Baldo). Mr Ryan Selleck was supported by training grant T32 GM007507 to the Neuroscience Training Program of the University of Wisconsin-Madison.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Selleck, R., Lake, C., Estrada, V. et al. Endogenous Opioid Signaling in the Medial Prefrontal Cortex is Required for the Expression of Hunger-Induced Impulsive Action. Neuropsychopharmacol 40, 2464–2474 (2015). https://doi.org/10.1038/npp.2015.97
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.97
This article is cited by
-
Delayed estrogen actions diminish food consumption without changing food approach, motor activity, or hypothalamic activation elicited by corticostriatal µ-opioid signaling
Neuropsychopharmacology (2023)
-
Dissociable control of μ-opioid-driven hyperphagia vs. food impulsivity across subregions of medial prefrontal, orbitofrontal, and insular cortex
Neuropsychopharmacology (2021)
-
Preconception paternal morphine exposure leads to an impulsive phenotype in male rat progeny
Psychopharmacology (2021)
-
Let’s call the whole thing off: evaluating gender and sex differences in executive function
Neuropsychopharmacology (2019)
-
Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone
Nature Communications (2019)