Abstract
The cerebellum is emerging as a key anatomical structure underlying normal attentional and cognitive control mechanisms. Dysregulation within cerebellar circuits may contribute to the core symptoms of Attention Deficit/Hyperactivity Disorder (ADHD). In the present study we aimed to characterize surface morphological features of the cerebellum in ADHD and healthy comparison youths. Further, we studied the association of cerebellar morphology with the severity of ADHD symptoms and the effects of stimulant treatment. We examined 46 youths with ADHD and 59 comparison youths 8–18 years of age in a cross-sectional, case–control study using magnetic resonance imaging. Measures of cerebellar surface morphology were the primary outcome. Relative to comparison participants, youths with ADHD exhibited smaller regional volumes corresponding to the lateral surface of the left anterior and the right posterior cerebellar hemispheres. Stimulant medication was associated with larger regional volumes over the left cerebellar surface, whereas more severe ADHD symptoms were associated with smaller regional volumes in the vermis. We used optimized measures of morphology to detect alterations in cerebellar anatomy specific to ADHD, dimensions of symptomology, and stimulant treatment. Duration of treatment correlated positively with volumes of specific cerebellar subregions, supporting a model whereby compensatory morphological changes support the effects of stimulant treatment.
Similar content being viewed by others
INTRODUCTION
Abnormalities in both morphology and functioning of fronto-striatal brain circuits have been linked to the pathogenesis of Attention Deficit/Hyperactivity Disorder (ADHD) (Durston et al, 2011). Recent reports have also suggested that ADHD is associated with perturbations in neuronal structures extending beyond fronto-striatal networks, including the thalamus (Ivanov et al, 2010) and cerebellum (Valera et al, 2007). The cerebellum in particular is emerging as a key structure in several neuropsychiatric conditions, including ADHD (Durston et al, 2011; O'Halloran et al, 2012). The functions of the cerebellum, previously considered limited to motor control, appear critical to higher cognitive processes, including learning (Steinlin, 2008), attention shifting (Akshoomoff et al, 1997; Courchesne et al, 1994; Golla et al, 2005), visual-spatial processing (Ivry, 1997; Ivry et al, 2002), working memory (Cooper et al, 2012; Stoodley et al, 2012), and emotion (Ferrucci et al, 2011; Schmahmann, 2004). Taken together, these prior findings suggest that the cerebellum could have an important role in the deficient attentional and cognitive control mechanisms present in ADHD.
The hypothesized role of the cerebellum in ADHD is further supported by observations that this structure has a protracted development, is sexually dimorphic, and is susceptible to environmental influences (Tiemeier et al, 2010). These observations are consistent with the clinical features of ADHD, which include an onset in early or mid-childhood when the cerebellum is still developing, its greater prevalence in boys, and the influence of environmental factors on the natural history of the disorder (Burt, 2009; Plomp et al, 2009). Lastly, the prefrontal cortex (PFC) and the basal ganglia are strongly interconnected with the cerebellum (Strick et al, 2009), thereby positioning the cerebellum to influence neural pathways previously implicated in ADHD.
Human neuroimaging studies have yielded evidence of reasonably consistent disturbances in the morphological characteristics of the cerebellum in individuals with ADHD (Seidman et al, 2005; Valera et al, 2007). First, a smaller size of the posterior cerebellar vermis (lobules VIII through X) has been consistently documented in ADHD for both males (Berquin et al, 1998; Bussing et al, 2002; Castellanos et al, 1996; Hill et al, 2003; Mostofsky et al, 1998) and females (Castellanos et al, 2001). The inferior posterior cerebellar vermis has been linked to poorer behavioral outcomes in ADHD (Kieling et al, 2008) and may account for a significant amount of the variance in ADHD symptoms (Bledsoe et al, 2011), findings that have led some to suggest that reduced volumes of the cerebellar vermis may represent a trait abnormality in ADHD (Mackie et al, 2007; Rapoport and Gogtay, 2008). Second, several studies have demonstrated reduced volumes of the cerebellar cortex in individuals with ADHD (Biederman et al, 2008; Castellanos et al, 1996; Castellanos et al, 2001; Castellanos et al, 2002; Mackie et al, 2007). Other studies suggest that volumes of the cerebellar cortex are influenced by maturational processes and that therefore volumetric differences present in childhood may resolve in adolescence (Castellanos et al, 2002; Shaw et al, 2006; Shaw et al, 2007).
Similar to other brain regions, the cerebellar hemispheres seem capable of morphological plasticity and hence may represent a potential target for clinical intervention (Mackie et al, 2007; Rapoport and Gogtay, 2008). This thesis is supported by one report documenting increased gray matter thickness in the right inferior posterior cerebellum following behavioral treatments (Hoekzema et al, 2011), and another showing that the vermis is of normal size in children with ADHD chronically treated with stimulants but smaller in those who are treatment-naive (Bledsoe et al, 2009). Taken together, these prior findings suggest a model of pathophysiology for ADHD in which volumetric reductions are localized to the cerebellar cortex and vermis but are also influenced by both maturational processes and treatment effects.
Recent advances in the processing of anatomical images permit the measurement of morphological features in the brain with improved precision and accuracy, permitting the detection of discrete differences in regional volumes of well-defined brain structures like the amygdala, hippocampus (Plessen et al, 2006), thalamus (Ivanov et al, 2010), basal ganglia (Sobel et al, 2010), and the cerebellum (Tobe et al, 2010). The current study employed one of these processing techniques, surface morphometry, to investigate in detail the purported differences in the cerebellar structure in youths with ADHD compared with control youths. Further, we studied the association of cerebellar morphology with both the severity of ADHD symptoms and the effects of stimulant treatment. We hypothesized that (1) youths with ADHD would have smaller regional cerebellar volumes compared with youths without ADHD, (2) smaller volumes would be associated with more severe ADHD symptoms, and (3) treatment with ADHD medication at the time of the scan would be associated with a relative normalization of regional cerebellar volumes.
MATERIALS AND METHODS
Participants
We studied a cohort of 105 children and adolescents, aged 8–18 years, divided into an ADHD (n=46) and a control group (n=59). This sample has been described elsewhere (Ivanov et al, 2010), characteristics of which are presented in Table 1. Written informed consent was obtained from all parents, and all children provided written assent. Diagnoses of ADHD were established using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (Kaufman et al, 1997) and a best-estimate consensus procedure that considered all available clinical and diagnostic information (Leckman et al, 1982). Socioeconomic status was estimated using the Hollingshead Four-Factor Index of Social Status (Hollingshead, 1975).
The presence of any lifetime or a current DSM-IV (American Psychiatric Association, 2000) Axis I disorder was exclusionary for the control group. Exclusion criteria for participants with ADHD were a lifetime diagnosis of any bipolar, psychotic, obsessive-compulsive, or tic disorders. Additional exclusion criteria for both groups were premature birth (gestation ⩽36 weeks), epilepsy, head trauma with loss of consciousness, lifetime substance abuse, developmental delay, or IQ below 80.
MRI Scanning and Image Analysis
Participants were scanned on a single 1.5-T scanner (GE Signa, Milwaukee). Head positioning was standardized using canthomeatal landmarks. T1-weighted sagittal three-dimensional volume images were obtained using a spoiled gradient echo pulse sequence (repetition time=24 msec, echo time=5 msec, flip angle=45°, matrix=256 × 192, field of view=30 cm, excitations=2, slice thickness=1.2 mm, contiguous slices=124) with spatial resolution of 1.17 × 1.17 × 1.2 mm3.
Preprocessing
ANALYZE 7.5 (Biomedical Imaging Resource, Mayo Foundation, Rochester, Minn.) running on SunUltra10 workstations was used for image processing. Operators were blind to cerebral hemisphere (images were randomly flipped left to right before analysis) and to clinical and demographic characteristics of the participants. Images were reformatted to a standard head position before region definition so that axial slices were oriented parallel to the anterior and posterior commissures and sagittal slices were oriented parallel to standard midline landmarks. Large-scale variations in image intensity were removed as described elsewhere (Ivanov et al, 2010). The cerebrum was isolated using an isointensity contour function in conjunction with manual editing. Conventional volumes were estimated using the distances in the axial, sagittal, and coronal planes to generate a single numerical measure for each participant.
Surface Morphometry
The cerebellum was defined using an isointensity contour function that separates the cerebellar cortex from the overlying cerebrospinal fluid. The cerebellar peduncles were transected from the brainstem manually in the coronal plane. The hemispheres were transected in the true cerebellar midline using a cubic spines plane. The distance from a voxel-sized point on the surface of each participant’s cerebellum to the corresponding point on the surface of the cerebellum in a template brain was calculated using previously validated methods (Bansal et al, 2005). We used a rigid-body similarity transformation with global scaling to register the entire brain of each participant with the template brain, which eliminated the need to further adjust for differences in whole-brain volume. The cerebellum of each participant was isolated and coregistered to the corresponding anatomy of a representative template cerebellum using a high-dimensional nonlinear warping based on fluid-flow dynamics that permitted the labeling of corresponding points across the surfaces of the cerebellum of each participant. The nonlinear warping was reversed to the refined registration to generate distances between individual points on the surface of each cerebellum and the corresponding points on the surface of the template cerebellum. Those distances provided measures of average indentations or protrusions on the cerebellar surface that were interpreted, respectively, as smaller or larger regional volumes and were then statistically analyzed using multiple linear regression at each voxel on the surface of the template cerebellum.
We applied a previously validated, two-step procedure to generate a cerebellar template that would be as representative as possible (in the sense of least-squares mean) to the average shape of the cerebellum for all healthy participants. First, we selected the cerebellum of the control subject who was most demographically representative of all healthy participants as a preliminary reference, and the cerebella for all other participants were then normalized to this preliminary reference. After determining the point correspondences on the template cerebellar surface, we computed the distance for all corresponding points between the template surface and the cerebellar surfaces for all controls. Second, the final template was selected as the cerebellum for which all points across its surface were closest, in terms of least-squares mean, to the average of all computed distances. We used a single representative template brain rather than an averaged brain from many youths, because the use of a single brain offers sharp borders at tissue interfaces and improves the accuracy of registration (Bansal et al, 2005; Plessen et al, 2006). In contrast, averaging images for a template blurs these boundaries and increases registration errors.
Volume Preserve Warping
We used volume preserved warping (VPW) to assess local expansion or reduction of tissue volumes below the cerebellar surface (Tobe et al, 2010). VPW preserves the intensity-weighted volume of each voxel during deformation by transforming the original intensity-weighted volumes into a normalized space and then comparing the intensities of corresponding brain regions within this normalized space. Thus, spatial normalization using VPW condenses relatively larger volumes so that they appear as voxels of relatively higher intensity values, whereas smaller volumes are expanded, appearing as voxels of relatively lower intensities (Xu et al, 2007; Xu et al, 2008). Analyses were carried out using in-house developed software.
Cytoarchetectonic Atlas
We overlaid on the template cerebellum a cythoarchetectonic atlas of the cerebellum to assist in the localization of findings from surface morphometry and VPW analyses (Supplementary Figure 4).
Statistical Analyses
Surface morphometry and VPW
The main effect of ADHD on imaging measures (surface distances or VPW voxels) was estimated by comparing those measures while covarying for age, sex, full scale IQ (FSIQ), and comorbidity (ie, this variable included comorbid ODD, depression, and anxiety disorders as described in Table 1). Three-way interactions between diagnosis and age, sex, and FSIQ were hierarchically ascertained using a mixed-model analysis. We corrected for the number of statistical comparisons in each model using the Gaussian Random Fields (GRFs) family-wise error method for surface morphometry and voxel-by voxel for VPW. Probability values are color-coded at each voxel and displayed across the template.
Associations with symptom severity
Within the ADHD group, we correlated cerebellar surface and VPW measures with symptom severity (ie, raw scores on the DuPaul-Barkley ADHD scale) using a general linear model that covaried for age and sex.
Medication effects
The effects of stimulant treatment were assessed within ADHD youths by comparing participants who received stimulants at the time of the scan vs youths who were not receiving medications at that time. We also correlated regional volumes with the duration of the stimulant treatment with β values calculated from the maxima voxel values (Supplementary Figure 5).
Conventional volumes
Statistical analyses were performed in SAS, version 9.0 (SAS Institute, Cary, NC). We tested our a priori hypothesis that conventional measures of overall cerebellar volume would differ across diagnostic groups by assessing the main effect of diagnosis in a mixed-models analysis with repeated measures. The model included the within-subjects factor ‘stimulants’ and ‘comorbidity’ with two levels (YES/NO) and the between-subjects factor of diagnosis (ADHD=1 and comparison subjects=0), and the covariates age, sex, FSIQ, and whole-brain volume. In addition, we considered three-way interactions of diagnosis, sex, FSIQ, and age. Statistically nonsignificant terms were eliminated via backward stepwise regression, with the constraint that the model at each step had to be hierarchically well-formulated (ie, all possible lower-order component terms of an interaction were included in the model, regardless of statistical significance). We considered P-values <0.05 statistically significant. All P-values were two-sided.
RESULTS
Demographics and Clinical Characteristics
The ADHD and control groups did not differ in age, FSIQ, or gender distribution (Table 1). Of the ADHD participants, 19 (41%) were diagnosed with one or more other psychiatric diagnoses, including oppositional defiant, depression, or anxiety disorders. However, in all cases, ADHD was judged to be the primary clinical problem at the time of scan. Of all ADHD participants, 31 (67%) were receiving stimulant medications at the time of the scan (range: 3–108 months, mean=43.3, SD±29.1). Five of the medicated participants received some additional psychotropic agent (Table 1). Of the 14 unmedicated participants, one had received stimulants in the past but was stimulant-free for 19 months at the time of scan. Results did not change when the five participants who received additional psychotropic medications other than stimulants, and when the participant who had received stimulants in the past but not at the time of the scan were removed from the analyses. The two ADHD subgroups (on stimulant vs off stimulant) did not differ in terms of age, gender, FSIQ, or ADHD symptom severity.
Surface Morphometry
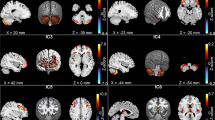
Relative to comparison participants, youths with ADHD exhibited significantly smaller regional volumes over the lateral surface of the left anterior and the right posterior cerebellar hemispheres localized to lobules IV, V, and VI, and the left crus I and right crus II (Figure 1). VPW analyses confirmed that the smaller volumes of these regions in ADHD participants derived from reduced volumes of the underlying tissue in these same regions. These effects were not accounted for by differences in age, gender, or FSIQ and we found no evidence of interaction between these variables. See Supplementary Figures 7 and 8 for analyses concerning age and gender.
Main effect of Attention Deficit/Hyperactivity Disorder (ADHD) diagnosis—Gaussian Random Fields (GRF) corrected. The figure shows statistical maps in different cerebellum views; the color bar indicates the color coding for P-values associated with the main effect of ADHD diagnosis, ranging from P<0.0001 in red (ie, increased regional volumes) and P<0.0001 in purple (ie, decreased regional volumes). The theory of Gaussian random field was used to correct for multiple comparisons. The maps show significantly smaller regional volumes in cerebellar lobules I–IV and crus I on the left as well as crus II on the right in youths with ADHD compared with healthy controls. L, left; R, right; VPW, volume preserve warping.
Medication Effects
Youths with ADHD who were receiving stimulant medications at the time of scan had significantly larger regional volumes over the left cerebellar surface compared with non-stimulant-treated ADHD youths localized in left lobules IV, V, VI, X, and right crus I and II. VPW analyses showed that the larger volumes of these regions in medicated ADHD participants derived from enlarged volumes of the underlying structures in these same regions. Although these regions closely overlapped with the finding of smaller regional volumes in ADHD compared with control youths, GRF analyses showed that smaller regional volumes associated with ADHD were localized more superiorly and laterally, whereas the larger volumes associated with stimulant treatment were localized more medially (lobule X, Figure 2). Duration of treatment correlated positively with regional volumes of the right Crus I and II and left lobule X (Figure 2b, also see Supplementary Figure 5). Additional surface morphometry analyses compare ADHD participants on and off stimulants and the controls (Supplementary Figure 9). The uncorrected figures show that ADHD participants off stimulants have smaller regional volumes in regions similar to those identified to show the main effect of diagnosis in the all ADHD participants vs control comparison. ADHD participants on stimulant, however, showed larger regional volumes in regions similar to those identified in the main effect of stimulant treatment analyses comparing ADHD on and ADHD off stimulants subgroups. These findings, however, did not survive FDR corrections.
Main effect of stimulant treatment—Gaussian Random Fields (GRF) corrected. The figure shows statistical maps in different cerebellum views; the color bar indicates the color coding for P-values associated with the main effect of stimulant treatment in subjects with Attention Deficit/Hyperactivity Disorder (ADHD) diagnosis (n=31), ranging from P<0.0001 in red (ie, increased regional volumes) and P>0.0001 in purple (ie, decreased regional volumes). The theory of the Gaussian random field was used to correct for multiple comparisons. The maps show significantly larger regional volumes in cerebellum lobule X on the left and crus II on the right in subjects with ADHD who were receiving stimulant treatment at the time of the scan compared with untreated subjects. L, left; R, right; VPW, volume preserve warping.
Correlations with Symptom Severity
More severe ADHD symptoms (total, inattentive, and hyperactivity) accompanied smaller regional volumes in the vermis (lobules VIIIa and VIIIb, Figure 3 and Supplementary Figure 6).
Correlations with Attention Deficit/Hyperactivity Disorder (ADHD) symptom severity—Gaussian Random Fields (GRF) corrected. The figure shows statistical maps in different cerebellum views; the color bar indicates the color coding for P-values correlated with the severity scores of ADHD symptoms in all subjects diagnosed with ADHD, ranging from P<0.0001 in red (ie, increased regional volumes) and P<0.0001 in purple (ie, decreased regional volumes). The theory of the Gaussian random field was used to correct for multiple comparisons. The maps show that significantly smaller regional volumes in the vermis VIIIa and VIIIb were accompanied by higher ADHD severity scores. L, left; R, right; VPW, volume preserve warping.
Conventional Volumes
We detected no main effect of group (F=0.58, df 1/98, P=0.45) and no significant effects of comorbid depression (F=0.31, df 2/94, P=0.73), ODD (F=1.94, df 2/96, P=0.15), or stimulant treatment (F=0.18, df 2/96, P=0.83) on conventional volumes (Table 2). The main effect of WBV indicates a significant scaling effect (eg, older individuals had larger WBV).
DISCUSSION
We detected significantly smaller regional volumes in the left anterior cerebellar hemisphere and right cerebellar crus in youths with ADHD compared with control youths. In contrast, treatment with stimulants was associated with an increased left anterior cerebellar volume compared with untreated ADHD youths. In addition, the severity of ADHD symptoms (total, hyperactivity, and inattention symptoms) correlated inversely with regional volumes in the anterior superior vermis, such that greater severity accompanied smaller volumes. Overall cerebellar volume did not differ significantly between ADHD and control groups.
ADHD and Morphological Abnormalities of the Cerebellum
Reduced cerebellar volumes in ADHD were localized to the left anterior lateral cerebellar hemisphere and the right cerebellar crus. The cerebellar hemispheres can be parsed functionally into a medial sector, the spinocerebellum, and a larger lateral sector, the cerebrocerebellum (Ghez and Fahn, 1985). The lateral zone, which in humans is by far the largest, receives input exclusively from the cerebral cortex (especially the parietal lobe) via the pontine nuclei (forming the cortico-ponto-cerebellar pathways) and sends projections to the ventrolateral thalamus, which is in turn connected to motor areas of the premotor cortex and primary motor area. Other brain regions connected to the lateral cerebellum, particularly the lateral thalamus, have been shown to be morphologically altered in youths with ADHD (Ivanov et al, 2010). The lateral cerebellum participates in evaluating sensory information and planning for action (Ghez and Fahn, 1985). Our findings, taken together with findings from these prior studies, suggest that ADHD may be associated with dysfunction within a distributed motor-control circuit involving the lateral cerebellum and the lateral thalamus. These morphological abnormalities are anatomically positioned to produce several of the important clinical features of ADHD, including behavioral impulsivity, impaired motor control, and poor sensory integration.
In contrast to previous reports, we did not detect a significant difference in overall cerebellar volume between ADHD and non-ADHD youths when controlling for age, gender, FSIQ, and whole-brain volume. The cerebellum is a large and anatomically heterogeneous structure comprising numerous substructures and nuclei, with pathological features in one or more substructures being diluted by the presence of normal tissue elsewhere in the cerebellum, making the overall volume of the cerebellum an insensitive and unreliable discriminator of group membership. In addition, our ADHD youths who received stimulants at the time of scan had regional enlargements compared with those not taking stimulants, which may have counterbalanced the smaller regional volumes in the ADHD group. Overall cerebellar volume seems to be a less informative measure than are local measures for the identification of morphological abnormalities associated with ADHD.
Symptom Severity and Cerebellar Morphology in ADHD
Decreased volume of the superior vermis was associated with greater ADHD symptom severity. The cerebellar vermis facilitates communication between the spinal cord and the cerebrum to maintain a balance between input from the peripheral nervous system and output from the central nervous system. Although we did not detect a main effect of ADHD diagnosis on vermis volumes, the severity correlations are generally consistent with the hypothesized role of the vermis in contributing to motor-control deficits in ADHD, which may in turn negatively influence attentional allocation.
ADHD has long been conceptualized as a disorder of the PFC and its connections, including the dorsal fronto-striatal circuits that subserve cognitive control, orbitofronto-striatal loops that support reward processing, and fronto-cerebellar networks that subserve timing functions, among others (Barkley, 1997). Neurobiological dysfunction in any of these circuits could contribute to the symptoms of ADHD. ADHD may be the product of a variety of processes that impair one’s capacity to allocate attention, control the intensity of motor activity, and monitor timing functions. Morphological abnormalities in different portions of the cerebellum (eg, vermis and lateral hemisphere regions) may produce differential effects on the behavioral phenotype, such as motor and perceptual timing that are also linked to attention and inhibitory deficits (Noreika et al, 2012). In turn, abnormalities in motor control may manifest as hyperactivity and altered time perception may contribute to the inability to tolerate delayed rewards resulting in impulsive actions.
Effects of Stimulants on Cerebellar Morphology
Treatment with stimulants was associated with an increased regional volume of the left lateral cerebellum, an effect in the direction of healthy control participants. We observed increased local volumes associated with stimulant use in regions other than the ones that were negatively affected by ADHD diagnosis. In other words, treatment did not ‘normalize’ morphological abnormalities linked to ADHD but rather increased regional volumes in neighboring structures, suggesting that the enlargement may in turn have attenuated ADHD symptoms via a compensatory function, rather than by reversing an underlying morphological abnormality. Such findings are in line with previous reports and meta-analyses showing that stimulant treatments influence brain morphology in other regions such as the left middle/inferior frontal gyrus, and the right parieto-occipital region (Shaw et al, 2009), the right lentiform and caudate nuclei (Nakao et al, 2011) basal ganglia (Sobel et al, 2010; Frodl and Skokauskas, 2012), and thalamus (Ivanov et al, 2010).
Our finding of increased regional volumes in the cerebellar hemisphere in treated participants is consistent with previous studies suggesting that the lateral cerebellum is capable of undergoing neuroplastic change, including plasticity associated with treatment (Durston et al, 2011). One study, for example, reported increases in gray matter of the cerebellum, among other regions, after cognitive training in individuals with ADHD, with the magnitude of increase associated with the degree of improvement in attention (Hoekzema et al, 2011). In addition, the volume of the vermis was found to be normal in chronically treated children but smaller in treatment-naive youths with ADHD (Bledsoe et al, 2009), whereas ADHD children with smaller cerebellar hemispheres have been reported to have poorer clinical outcomes (Mackie et al, 2007). Others have shown that an acute dose of methylphenidate increases (Epstein et al, 2007) and potentially normalizes functional activity of the cerebellum in youths with ADHD (Hart et al, 2012). Stimulants could increase dopamine levels within the dense dopamine projections to the cerebellum, which in turn could increase the density of dendritic spines and subsequently local volumes of the cerebellar cortex. The finding that the duration of treatment in our sample correlated positively with regional hemisphere volumes (eg, longer the treatment periods were associated with larger volumes) further suggests that dopaminergic influences may contribute to cerebellar plasticity. The mechanisms underlying treatment-related plasticity, however, are speculative and require further study.
Limitations and Conclusions
This study has several limitations. First, its cross-sectional design precludes definitive conclusions regarding causality between stimulant treatment and observed morphological differences between treated and untreated ADHD youths. Longitudinal studies are needed to establish more definitively the relationship between cerebellar morphology and treatment effects. Second, analyses of the cerebellar surface assign the source of morphological differences to the most superficial tissues, even when these differences may derive instead from abnormalities in deeper underlying nuclei. This shortcoming, however, was mitigated by the use of VPW, which assessed morphology throughout the three-dimensional volume of the cerebellum, supporting the localization of findings primarily to superficial locations. Another study limitation is that males are over-represented in our sample, thereby limiting our ability to detect gender effects. Although we did not find significant effects of gender after controlling for multiple comparisons, future studies utilizing more balance samples will be needed to draw definitive conclusions. In summary, our results confirm that structural deficits in the cerebellar hemispheres and the vermis are associated with childhood ADHD and symptom severity. Further, stimulant treatment was associated with enlargement in specific portions of the cerebellar hemispheres that were distinct from the regions of reduced volume associated with a diagnosis of ADHD. These enlargements correlated positively with treatment duration, supporting a model whereby compensatory morphological changes support the effects of stimulant treatment.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Akshoomoff NA, Courchesne E, Townsend J (1997). Attention coordination and anticipatory control. Int Rev Neurobiol 41: 575–598.
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association: Washington, DC, USA.
Bansal R, Staib LH, Whiteman R, Wang YM, Peterson BS (2005). ROC-based assessments of 3D cortical surface-matching algorithms. Neuroimage 24: 150–162.
Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121: 65–94.
Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL et al (1998). Cerebellum in attention-deficit hyperactivity disorder: a morphometric MRI study. Neurology 50: 1087–1093.
Biederman J, Makris N, Valera EM, Monuteaux MC, Goldstein JM, Buka S et al (2008). Towards further understanding of the co-morbidity between attention deficit hyperactivity disorder and bipolar disorder: a MRI study of brain volumes. Psychol Med 38: 1045–1056.
Bledsoe J, Semrud-Clikeman M, Pliszka SR (2009). A magnetic resonance imaging study of the cerebellar vermis in chronically treated and treatment-naive children with attention-deficit/hyperactivity disorder combined type. Biol Psychiatry 65: 620–624.
Bledsoe JC, Semrud-Clikeman M, Pliszka SR (2011). Neuroanatomical and neuropsychological correlates of the cerebellum in children with attention-deficit/hyperactivity disorder—combined type. J Am Acad Child Adolesc Psychiatry 50: 593–601.
Burt SA (2009). Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychol Bull 135: 608–637.
Bussing R, Grudnik J, Mason D, Wasiak M, Leonard C (2002). ADHD and conduct disorder: an MRI study in a community sample. World J Biol Psychiatry 3: 216–220.
Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T et al (2001). Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 58: 289–295.
Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP et al (1996). Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry 53: 607–616.
Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS et al (2002). Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 288: 1740–1748.
Cooper FE, Grube M, Von Kriegstein K, Kumar S, English P, Kelly TP et al (2012). Distinct critical cerebellar subregions for components of verbal working memory. Neuropsychologia 50: 189–197.
Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ et al (1994). Impairment in shifting attention in autistic and cerebellar patients. Behav Neurosci 108: 848–865.
Durston S, van Belle J, de Zeeuw P (2011). Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biol Psychiatry 69: 1178–1184.
Epstein JN, Casey BJ, Tonev ST, Davidson MC, Reiss AL, Garrett A et al (2007). ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J Child Psychol Psychiatry 48: 899–913.
Ferrucci R, Giannicola G, Rosa M, Fumagalli M, Boggio PS, Hallett M et al (2011). Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cogn Emot 26: 786–799.
Frodl T, Skokauskas N (2012). Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand 125: 114–126.
Ghez C, Fahn S (1985). The cerebellum. In: Kandel E.R, Schwartz JH (eds) Principles of Neural Science. Elsevier: New York, NY, USA. pp 502–522.
Golla H, Thier P, Haarmeier T (2005). Disturbed overt but normal covert shifts of attention in adult cerebellar patients. Brain 128: 1525–1535.
Hart H, Radua J, Mataix-Cols D, Rubia K (2012). Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD). Neurosci Biobehav Rev 36: 2248–2256.
Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, Brooks W (2003). Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology 17: 496–506.
Hoekzema E, Carmona S, Ramos-Quiroga JA, Barba E, Bielsa A, Tremols V et al (2011). Training-induced neuroanatomical plasticity in ADHD: a tensor-based morphometric study. Hum Brain Mapp 32: 1741–1749.
Hollingshead AB (1975) Four-Factor Index of Social Status. Yale University: New Haven, CT, USA.
Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L et al (2010). Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry 167: 397–408.
Ivry R (1997). Cerebellar timing systems. Int Rev Neurobiol 41: 555–573.
Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J (2002). The cerebellum and event timing. Ann N Y Acad Sci 978: 302–317.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36: 980–988.
Kieling C, Goncalves RR, Tannock R, Castellanos FX (2008). Neurobiology of attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin N Am 17: 285–307 viii.
Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM (1982). Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry 39: 879–883.
Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF 3rd et al (2007). Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry 164: 647–655.
Mostofsky SH, Reiss AL, Lockhart P, Denckla MB (1998). Evaluation of cerebellar size in attention-deficit hyperactivity disorder. J Child Neurol 13: 434–439.
Nakao T, Radua J, Rubia K, Mataix-Cols D (2011). Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry 168: 1154–1163.
Noreika V, Falter CM, Rubia K (2012). Timing deficits in attention-deficit/hyperactivity disorder (ADHD): Evidence from neurocognitive and neuroimaging studies. Neuropsychologia 51: 235–266.
O'Halloran CJ, Kinsella GJ, Storey E (2012). The cerebellum and neuropsychological functioning: a critical review. J Clin Exp Neuropsychol 34: 35–56.
Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA et al (2006). Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 63: 795–807.
Plomp E, Van Engeland H, Durston S (2009). Understanding genes, environment and their interaction in attention-deficit hyperactivity disorder: is there a role for neuroimaging? Neuroscience 164: 230–240.
Rapoport JL, Gogtay N (2008). Brain neuroplasticity in healthy, hyperactive and psychotic children: insights from neuroimaging. Neuropsychopharmacology 33: 181–197.
Schmahmann JD (2004). Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 16: 367–378.
Seidman LJ, Valera EM, Makris N (2005). Structural Brain Imaging of Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry 57: 1263–1272.
Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D et al (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA 104: 19649–19654.
Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A et al (2006). Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 63: 540–549.
Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS et al (2009). Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry 166: 58–63.
Sobel LJ, Bansal R, Maia TV, Sanchez J, Mazzone L, Durkin K et al (2010). Basal ganglia surface morphology and the effects of stimulant medications in youth with attention deficit hyperactivity disorder. Am J Psychiatry 167: 977–986.
Steinlin M (2008). Cerebellar disorders in childhood: cognitive problems. Cerebellum 7: 607–610.
Stoodley CJ, Valera EM, Schmahmann JD (2012). Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59: 1560–1570.
Strick PL, Dum RP, Fiez JA (2009). Cerebellum and nonmotor function. Annu Rev Neurosci 32: 413–434.
Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN (2010). Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage 49: 63–70.
Tobe RH, Bansal R, Xu D, Hao X, Liu J, Sanchez J et al (2010). Cerebellar morphology in Tourette syndrome and obsessive-compulsive disorder. Ann Neurol 67: 479–487.
Valera EM, Faraone SV, Murray KE, Seidman LJ (2007). Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry 61: 1361–1369.
Xu D, Hao X, Bansal R, Plessen KJ, Geng W, Hugdahl K et al (2007). Unifying the analyses of anatomical and diffusion tensor images using volume-preserved warping. J Magn Reson Imaging 25: 612–624.
Xu D, Hao X, Bansal R, Plessen KJ, Peterson BS (2008). Seamless warping of diffusion tensor fields. IEEE Trans Med Imaging 27: 285–299.
Acknowledgements
This project was supported by grants NIMH K0274677, NIMH MH068318, MH59139, the Tom Klingenstein and Nancy Perlman Family Fund, and the Suzanne Crosby Murphy endowment at Columbia University. Dr Ivanov has received honorarium from Lundbeck unrelated to the current report and has also received travel support from the American Psychological Association, American College of Psychopharmacology, Alcohol Medical Scholar Program and American Professional Society of ADHD and Related Disorders. Dr Murrough is supported by a Career Development Award from the NIMH (K23MH094707) and receives additional research support from the American Foundation for Suicide Prevention, the Doris Duke Charitable Foundation, Janssen Research & Development and Avanir Pharmaceuticals. Dr Peterson has received investigator-initiated research support from Eli Lilly and Pfizer. The work contained herein is solely the responsibility of the authors and does not necessarily reflect the views of the NIH, NIMH, or other funding body.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Ivanov, I., Murrough, J., Bansal, R. et al. Cerebellar Morphology and the Effects of Stimulant Medications in Youths with Attention Deficit-Hyperactivity Disorder. Neuropsychopharmacol 39, 718–726 (2014). https://doi.org/10.1038/npp.2013.257
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.257
Keywords
This article is cited by
-
The ability to maintain rhythm is predictive of ADHD diagnosis and profile
BMC Psychiatry (2023)
-
Structural and functional deficits and couplings in the cortico-striato-thalamo-cerebellar circuitry in social anxiety disorder
Translational Psychiatry (2022)
-
Abnormal hemispheric asymmetry of both brain function and structure in attention deficit/hyperactivity disorder: a meta-analysis of individual participant data
Brain Imaging and Behavior (2022)
-
Viewing the Personality Traits Through a Cerebellar Lens: a Focus on the Constructs of Novelty Seeking, Harm Avoidance, and Alexithymia
The Cerebellum (2017)
-
The Cerebellum and Neurodevelopmental Disorders
The Cerebellum (2016)