Abstract
The combination of pharmacotherapy and cognitive retraining (CRT) for the cognitive deficits of schizophrenia may be more efficacious than either approach alone, but this has not yet been tested. This study evaluated the feasibility, safety, tolerability, and efficacy of 12 weeks of D-serine, combined with CRT in the treatment of cognitive deficits in schizophrenia at two academic sites in parallel, in India and the United States. In a randomized, partial double-blind, placebo-controlled, parallel-group design, 104 schizophrenia subjects (US site=22, Indian site=82) were randomized to: (1) D-serine (30 mg/kg)+CRT (5 h/week), (2) D-serine+control CRT, (3) CRT+placebo D-serine, and (4) placebo+control CRT. Completion rates were 84 and 100% in the Indian and US samples, respectively. On various outcome measures of safety and tolerability, the interventions were well tolerated. D-Serine and CRT did not show any significant effect on the Global Cognitive Index, although both interventions showed differential site effects on individual test performance. CRT resulted in a significant improvement in Verbal Working Memory, and a trend toward improvement in Attention/Vigilance. This is the first study to demonstrating the feasibility, safety, and tolerability of combination pharmacotherapy and CRT in a multicenter international clinical trial. These preliminary findings provide support for future studies using higher doses of D-serine that have been shown to be efficacious or other pharmacotherapies, along with the newer cognitive remediation strategies that are individualized and that target basic information processing.
Similar content being viewed by others
INTRODUCTION
There is a need to develop new treatments for schizophrenia. Existing antipsychotic drugs, all of which block D2 receptors, continue to be the mainstay of treatment (Kapur and Mamo, 2003). Dopamine D2 receptor antagonists have limited efficacy for negative symptoms and cognitive deficits (Buchanan et al, 2007; Keefe et al, 2007). Cognitive deficits affect most patients with schizophrenia (Keefe et al, 2005), range from moderate to severe (Heinrichs and Zakzanis, 1998), and are strongly correlated with functional outcome (Green et al, 2000). In fact, the degree of neurocognitive deficits is a better predictor of disability and vocational functioning than positive symptoms (Tsang et al, 2010). Currently, the strategies that are being developed to target cognitive deficits broadly consist of pharmacological agents and cognitive retraining (CRT).
A number of putative pharmacological cognitive enhancers are being tested in schizophrenia (Arnsten et al, 1994; Bradley et al, 2010; Homayoun and Moghaddam, 2010; O’Donnell et al, 2010; Radek et al, 2010). It may be the case that when patients are studied in the context of the impoverished level of cognitive stimulation that is typical of schizophrenia and that is not conducive to experience-dependent plasticity, it will be difficult to detect any cognitive enhancement from experimental pharmacotherapies. Thus, just as physical exercise is necessary to realize the benefits of anabolic steroids, cognitive exercise might be necessary to realize the benefits of cognitive enhancers (Keefe et al, 2011).
CRT refers to ‘behavioral training based intervention that aims to improve cognitive processes’. CRT has been shown to produce modest improvements in cognition with modest durability in the domains of attention, speed of processing, problem solving, and social cognition (McGurk et al, 2007b) in patients receiving antipsychotic treatment. CRT has also been shown to help in the acquisition of new skills and generalizes to work outcome (McGurk et al, 2007a, 2007b).
Although CRT and psychopharmacological approaches to enhance cognitive function have been used separately, to our knowledge they have not been used in combination, even though, as discussed below, the combination is likely to be more effective than either strategy alone.
Several lines of evidence suggest that deficits in NMDA receptor function contribute to the neurobiology of schizophrenia (Coyle and Tsai, 2004; Javitt, 2007). Furthermore, NMDA receptors play an important role in neuronal plasticity (Rebola et al, 2010). The significant role played by NMDA glutamate receptors in both the pathophysiology of schizophrenia (Krystal et al, 2003) and neuronal plasticity (Rebola et al, 2010) suggest that facilitation of NMDA receptor function might address the cognitive deficits in schizophrenia. Stimulation of the co-agonist glycine allosteric site on the NMDA receptor offers one method to enhance NDMA receptor function. NMDA receptor function can be safely enhanced either directly with agonists of the NMDA receptor-associated glycine site such as glycine and D-serine or indirectly with glycine transporter inhibitors. There is growing evidence that D-serine is an important endogenous ligand at the NMDA receptor glycine site (Gustafson et al, 2007) and disturbances in D-serine metabolism have been reported in schizophrenia (Kantrowitz et al, 2010). Augmentation of antipsychotic treatment with glycine-like drugs or glycine transporter inhibitor has shown modest effects on a range of symptoms associated with schizophrenia (Javitt, 2009). D-Serine, by facilitating NMDA receptor function and enhancing synaptic plasticity, would be expected to maximize the benefits that schizophrenic patients could derive from computerized CRT.
The primary aim of this study was to determine the feasibility and safety of combining CRT and D-serine in a multisite trial, and the secondary aim was to determine the efficacy of the combination.
PATIENTS AND METHODS
Setting
The study was a mixed, double-blind, placebo-controlled, parallel-group design and was conducted at two international sites: (1) VA Connecticut Healthcare System, West Haven, Connecticut, USA and (2) National Institute of Mental Health and NeuroSciences (NIMHANS), Bangalore, India.
Approvals
The study was conducted with the approval of the relevant regulatory bodies in India and the United States (see Supplementary Text). Written informed consent was obtained from each patient in English or the main local languages, Kannada and Hindi. The consent process included a questionnaire that subjects had to pass to ensure that they understood the key risks and benefits of the study. Efforts were made to involve family members, the treating clinician, and, where relevant, an ombudsperson in the consent process.
Sample
For both sites, male and female patients diagnosed with DSM-IV schizophrenia or schizoaffective disorder, aged 18–65 years with at least primary-school education (8 years), were included. For the India site, only English-, Hindi- or Kannada-speaking subjects were included and at the US site only English-speaking subjects were included. Patients were required to be medically healthy and clinically stable as determined by their primary clinician, to have been treated with antipsychotic medications for at least 6 months, to be on a stable dose of the same antipsychotic medication over the past 1 month, and able to give informed consent. Patients who had been recently hospitalized, or had required an increase in antipsychotic medications, were excluded. Patients with screening Calgary Depression Scale (CDS) score >10 and Simpson-Angus Neurological Rating Scale (NRS) score >20 were excluded to minimize the likelihood of significant depression and/or extrapyramidal symptoms interfering with the assessment of negative symptoms and cognitive deficits. Subjects taking lamotrigine, carbamazepine, or clozapine, and those who were treatment refractory were excluded because these drugs may interfere with the effect of D-serine on facilitating NMDA receptor function (Javitt et al, 2005; Lee et al, 2008; Ninan et al, 2003; Giustizieri et al, 2008). Subjects with IQ <70, abnormal thyroid function, and recent (3 months) risk of suicide or substance abuse or dependence (except for nicotine) were also excluded. Women of child-bearing potential were required to have a negative pregnancy test, to be using an acceptable method of contraception, and not be pregnant or lactating. Antipsychotic dose and brand could not be changed during the course of the study. Anticholinergic medications and benzodiazepines were withheld for 12 h before cognitive testing to minimize potential acute effects on cognitive assessments.
Randomization
After an extensive screening process (Supplementary Text), eligible subjects were randomized to receive: (1) D-serine plus CRT, (2) placebo D-serine plus CRT, (3) D-serine plus control CRT (video viewing), or (4) placebo D-serine plus control CRT for 12 weeks. Randomization was stratified by screening performance IQ (71–80, 81–90, 91–100, and >100). Separate randomization schedules with a block size of 4 were generated within each IQ stratum.
Duration
The treatment phase lasted 12 weeks, followed by an additional 24 weeks to determine the durability of any effects of the intervention.
Drugs
Subjects received D-serine (30 mg/kg) or placebo for 12 weeks. D-Serine was obtained from Degussa, Paris, France at >99.1% purity and packaged by the VA Cooperative Studies Program Clinical Research Pharmacy (Albuquerque, NM) into 200 and 500 mg capsules along with matching placebo. D-Serine was tested every 6 months and remained well within the potency acceptance criteria of 85–115%.
Blinding
The drug condition was double blind. The CRT condition was single blind with the rater, but not the subject, being kept blind to whether the subject was receiving CRT or control CRT.
Cognitive Retraining
Subjects were randomized to computerized or control CRT for 5 h per week spread across 2–3 days/week under the supervision of a clinical psychologist and research physician in the Neurocognitive Retraining Laboratory. For the control CRT condition, subjects watched noninteractive, neutral videos of popular local TV programs. The computerized CRT module consisted of 20 computer-assisted tasks (4 tasks per cognitive domain) targeting attention, memory, verbal and visuospatial working memory, and executive function that were derived from the Psychological Software Services CogRehab software (Chen et al, 1997) (detailed in Supplementary Text).
Payment
Subjects were paid for participation in the study. Subjects in the US sample received $100.00 after screening and $200.00 at the end of the study. In addition, subject received $3.40 per hour for the active or control CRT sessions. Therefore, subjects in the US sample received a total of $504.00 for participating in all study procedures. Subjects in the Indian sample were paid taking into account the prevailing socioeconomic conditions and research culture at NIMHANS. Thus, subjects in the Indian sample were paid Rs 300 at screening and Rs 600 at the end of the treatment phase, Rs 600 per week for completing 5 h per week of the active or control CRT sessions, and Rs 300 each for the two follow-up visits. Therefore, subjects in the Indian sample received a total of Rs 8700 (∼$175) for participating in all study procedures.
Outcome Measures
As detailed in Supplementary Table 1, outcomes were measured at baseline, at several times during the active treatment phase, and at the follow-up phase.
Feasibility Outcomes
The key indicators to evaluate the feasibility of this study were the rate of enrollment, retention of patients, number of training sessions completed during the course of the trial, and completion rate of primary outcome.
Safety Outcomes
Psychosis and depression were assessed using the Positive and Negative Syndrome Scale (PANSS)(Kay et al, 1989) and Calgary Depression Scale (Addington et al, 1990), respectively, at weeks 0, 2, 4, 8, and 12 of the active treatment phase of the study and at the 1-, 3-, and 6-month time points during follow-up. Side effects were assessed using the NRS; Simpson and Angus, 1970), Barnes Akathisia Rating Scale (BARS; Barnes, 1989), Abnormal Involuntary Movement Scale (AIMS; Guy, 1976 (reprinted 1991)), and Udvalg for Kliniske Undersogelser (UKU) Side Effects Rating Scale (Lingjaerde et al, 1987). Change in renal function, as evidenced by a 30% increase in serum creatinine, was monitored as changes in renal function have been reported in rats with very high doses of D-serine.
Cognitive Efficacy Measures
Among the cognitive outcome measures, attention was assessed using Continuous Performance Test (CPT; Gordon, 1986) similar to the AX-CPT; speed of processing was measured using WAIS-III Digit Symbol coding and Trail Making Test, part A; verbal working memory was measured using WAIS-III Digit Span and WAIS-III Letter/Number Sequencing; visual working memory was assessed using WAIS-III Spatial Span; memory was assessed using the Hopkins Verbal Learning Test-Revised (HVLT-R) and WAIS- III Logical Memory; and executive functioning was assessed using the Tower of London (TOL) and Wisconsin Card Sorting Test (WCST; Supplementary Table 2).
Functional Outcome Measures
These included Social Skills Performance Assessment (Patterson et al, 2001b), Medication Management Ability Assessment (Patterson et al, 2002), University of California San Diego (UCSD) Performance-Based Skills Assessment (UPSA) (Patterson et al, 2001a), and Heinrichs-Carpenter Quality-of-Life Scale (QOL) (Heinrichs et al, 1984). The HLVT-R and UPSA were modified for the Indian site to make them relevant to the Indian context (detailed in Supplementary Text).
Statistics
The primary outcome for this trial was determination of the feasibility, safety, and tolerability of conducting a multisite trial using D-serine, completion rate, and cognitive retraining during the treatment phase (baseline to week 12). The z-scores of the Indian and US samples were calculated separately at all time points because of significant between-site heterogeneity (Table 1 and Supplementary Table 3d). Each outcome was tested for normality using Kolmogorov–Smirnov test statistics and normal probability plots. A Global Cognitive Index (GCI) was computed using data from tests that tapped into different cognitive domains. The tests included Trails A, WAIS-III Digit Symbol coding (Speed of Processing), Hopkins Verbal Learning Test, WAIS-III Logical Memory-immediate recall (Verbal Learning and Memory), Continuous Performance Test (CPT-d′) (Sustained Attention domain), letter-number sequencing and spatial span (Working Memory), and WCS, and TOL (Reasoning and Problem solving). Missing data were handled using last-observation-carried-forward (LOCF) method for weeks 4, 8, and 12, and mean imputation in case of missing baseline data. The z-scores were then summed to derive the GCI (Raw data in Supplementary Tables 9.i–9.iii).
Factorial ANOVA with D-serine (placebo vs active), CRT (video vs active), and Time (week 0 to week 12) as fixed effects was used to evaluate GCI as the outcome variable, and the interactions between Site, Time, D-serine, and CRT were tested. Significant interactions were interpreted using post hoc tests (ie, comparing CRT effects within each level of D-serine, and vice versa) and graphical displays. Data were analyzed using SAS, version 9.1 (SAS Institute, Cary, NC). All results were considered statistically significant at P<0.05.
RESULTS
Enrollment
Subjects were enrolled between 2003 and 2008, with enrollment of subjects in India starting later (2005) and ending in 2008 (Supplementary Table 10).
Demographics
The four treatment groups within each site did not differ at baseline on any of the demographic and clinical outcome measures (Supplementary Tables 3a, 3b, 3c, 6, and 7). However, there were statistically significant differences in age, weight, marital status, handedness, and PANSS scores of subjects between the two sites (Table 1). There were also significant (all P-values <0.05) between-site baseline differences on a number of cognitive and functional measures (Supplementary Table 3d).
Feasibility
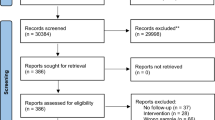
Of the 132 subjects (US site=36, Indian site=96) who were screened, 104 subjects were randomized as illustrated in the Consort diagram (Figure 1). In the Indian sample, 69 of 82 (84%) completed the 12-week active treatment phase and 43/82 (52%) completed the 6-month follow-up, whereas in the US sample, 22/22 (100%) completed the treatment phase and 21/22 (95.5%) completed the follow-up phase. The number of subjects who dropped out in the Indian sample were n=2, n=5, n=3, and n=3 in the D-serine+placebo CRT, CRT+placebo D-serine, D-serine+CRT, and placebo conditions, respectively. Of the 13 noncompleters, 8 were unable to maintain the time commitment, 1 reported exacerbation of psychotic symptoms, 2 reported abdominal discomfort, and 2 withdrew for unspecified reasons.
Consort chart.
Study Completion Rates
There were no significant differences in completion rates among the four groups during the active treatment phase (χ2(3)=1.59, p=0.66).
Completion of Cognitive Efficacy Measures
For the primary efficacy measures, 90.9% (±5.7) of data were available from the Indian sample across all the cognitive assessments and 100% (±0%) from the US sample. Less than 5% of data were missing at baseline because some assessments could not being completed due to conflicting demands of the baseline visit. For missing baseline data, the site means were imputed; the latter were used because of between-site baseline differences.
Number of Cognitive Retraining Sessions Completed
Among those who completed the 12-week active treatment phase, the rates of completion of cognitive retraining sessions were 100% at both sites.
Safety/Tolerability
There were no significant differences among the four treatment group overall symptoms (PANSS Total and all subscales) and depression (Calgary Depression Scale), either in the treatment phase (0–12 weeks) (Table 2a) or in the follow-up phase (12–36 weeks), and on extrapyramidal symptoms (NRS) in the treatment phase. Akathisia scores (BARS) decreased from baseline to 12 weeks with D-serine in the Indian sample (p=0.07; D-serine × site × time interaction F(1, 96)=4.13, p=0.04) whereas the CRT+placebo arm showed an increase in abnormal involuntary movements in the US sample (p=0.02; CRT × D-serine × site × time interaction F(1, 96)=4.09, p=0.046; CRT × D-serine × time interaction F(1, 96)=3.98, p=0.049; Table 2a), but these were very small effects of uncertain clinical significance. There were no significant medication-related side effects (UKU Side Effects Rating Scale) between D-serine and placebo (patient-rated global assessment of interference χ2(1)=1.21, p=0.27; physician-rated global assessment of interference χ2(1)=0.26, p=0.61) at the 12-week time point. Laboratory testing conducted at weeks 0, 4, 8, and 12 did not show any clinically significant changes. In the Indian sample, three subjects reported abdominal discomfort, which was judged to be possibly related to study medication, and one subject experienced an exacerbation of psychosis after having missed a dose of depot neuroleptics.
Cognitive Efficacy Outcome Measures
Global cognition index
There was no significant change in cognition as measured by the GCI in any of the four arms over the active treatment phase (Table 2bi). In the factorial ANOVA, there were no significant main or interactive effects of D-serine, CRT, site, or time on the GCI, although there was a drug × CRT × site × time interaction at a trend level of significance (F(1, 96)=3.51, p=0.06), with no significant post hoc comparisons.
Individual cognitive test performance data.
D -Serine effects: On the Spatial SpanTotal, a measure of Nonverbal Working Memory, D-serine showed a differential Site effect over time (D-serine × site × time interaction F(1, 96)=4.34, p=0.04) (Table 2bii). This was mediated by a worsening in performance with D-serine in the Indian sample and an improvement in performance in the US sample, although post hoc comparisons were not significant.
CRT effects: CRT was associated with a significant effect on ‘Verbal Working Memory’ as measured by Digit Span-Forward score (CRT × time interaction (F(1, 96)=3.60, p=0.049)). This effect was mediated by an improvement in the CRT group and a worsening in the group that received placebo CRT, although post hoc comparisons were not significant.
CRT was also associated with a trend (D-serine × CRT × time interaction (F(1, 96)=3.81, p=0.05)) towards improvement in ‘Attention/Vigilance’ as measured on the CPT-d′ in the group that received placebo D-serine (post hoc p=0.077). There was a significant effect of site (D-serine × CRT × site × time interaction F(1, 96)=3.75, p=0.056), with the US sample showing significant improvements between week 12 and baseline (p=0.008) and the India sample showing numerical worsening that did not reach statistical significance. Irrespective of D-serine treatment, there was also an effect of site (CRT × site × time interaction (F(1, 96)=8.54, p=0.004)) driven by the two sites showing divergent responses. However, the improvements in the US sample and the worsening in the Indian sample did not reach statistical significance.
Interactions between D -Serine and CRT: D-Serine did not increase the effects of CRT as measured on cognitive test performance.
Follow-Up Comparison Between Weeks 12 and 36
Global cognitive index
Only 52% of the sample completed the 36-week follow-up. Using a LOCF approach, at the 36-week follow-up, the group that received D-serine trended to have higher scores on the GCI compared with week 12 (D-serine × time interaction (F(1, 96)=3.56, p=0.06)). Furthermore, there was a differential site effect (D-serine × site × time interaction (F(1, 96)=4.23, p=0.04)), with the two sites showing divergent responses; however, the performance improvements in the US sample and the decline in the Indian sample did not reach statistical significance.
Functional Outcome Measures
There were no significant effects for D-serine, CRT, or D-serine × CRT interaction over time on any of the functional outcome measures except for trend-level effects of CRT over time on the Social Skills Performance Assessment (F(1, 96)=3.25, p=0.07) mediated by an improvement on Social Skills performance in the group that received CRT and a worsening in the group that received placebo CRT (Table 2c). However, these changes did not reach the level of statistical significance on post hoc comparisons.
Medication Management Ability Assessment data revealed a significant site × D-serine interaction over time (site × D-serine × time; F(1, 96)=5.83, p=0.02) mediated by a worsening in the D-serine group in the US sample and an improvement in the D-serine group in the Indian sample, although there were no significant post hoc comparisons (Table 2c).
D-Serine Levels
D-serine treatment was associated with higher plasma D-serine levels over 12 as described in the Supplementary section and Supplementary Figure 1.
DISCUSSION
To our knowledge, this is the first randomized, controlled study investigating the feasibility, safety, tolerability, and efficacy of the combination of a pharmacological and cognitive retraining approach to ameliorate cognitive dysfunction in schizophrenia. Furthermore, this is the first study, to our knowledge, combining CRT and D-serine with ongoing antipsychotic medication in the treatment of cognitive symptoms of schizophrenia.
Feasibility
The time commitment for subjects was considerable. Subjects had to travel to the clinic at least 2 times a week for several hours per visit. This was particularly challenging for subjects in Bangalore, India, who faced the traffic problems of a growing metropolis. Furthermore, subjects were repeatedly subjected to a battery of clinical and cognitive assessments. Despite this, 84% of subjects completed the treatment phase, 100% of subjects completed the cognitive training sessions, and >90% completed the cognitive efficacy assessments during the treatment phase, suggesting that subjects did not seem to find the time commitment, the assessments, or the training burdensome. These completion rates are comparable to other cognitive retraining trials and pharmacological trials for cognition in schizophrenia (Klingberg et al, 2011; Wykes and Spaulding, 2011), suggesting that the combination of CRT and pharmacological intervention should not significantly affect recruitment, retention, or study completion. A number of factors that may have contributed to the high completion rates during the treatment phase include, but are not limited to, the possibility of receiving a new pharmacotherapy and novel nonpharmacological treatment, and payment for study visits.
In terms of training of study personnel, enrollment, patient engagement, and study completion, the results of this study suggest that transcontinental, multisite trials of combined cognitive retraining and pharmacotherapy are feasible. In contrast to the treatment phase, only 52% of subjects completed the follow-up phase in the Indian sample. This raises questions about the feasibility of the follow-up phase. There were no significant differences between completers and noncompleters on cognitive measures at baseline or week 12 (Supplementary Tables 8i and ii). The most likely explanation for the high dropout rate is that the travel burden for the follow-up visits exceeded the benefits. The end of treatment and the lower cumulative compensation for the follow-up visits relative to the treatment phase may have reduced the incentive for subjects to return. Furthermore, the duration between follow-up visits compared with the weekly treatment visits may have promoted disengagement. Relative to the US site, subjects in India had greater challenges traveling to appointments. The travel time ranged from 2 to 4 h per visit and in several instances subjects traveled with family members from nearby towns and villages the night before and stayed over at the NIMHANS guest house to be on time for appointments. Given the importance of determining how durable the effects of combination pharmacotherapy and CRT, future studies will need to devise strategies to reduce attrition.
Safety and Tolerability
The combination of D-serine and CRT, D-serine+placebo CRT, and CRT+placebo D-serine was safe and well tolerated as reflected in no significant worsening in measures of psychosis, depression, extrapyramidal symptoms, drug side effects, and laboratory indices. The latter add to the limited data on the renal safety of D-serine in humans.
Efficacy
D-Serine, CRT, or the combination of the two did not show any significant effect on the GCI. The lack of improvement in cognitive test performance with D-serine may be related to the dose of D-serine used in this study (30 mg/kg), as higher doses (60 mg/kg) have been shown to be improve cognitive test performance (Kantrowitz et al, 2010). However, at the time this study was conceived, the known safety of D-serine limited the dose that could be administered safely to humans to 30 mg/kg. D-Serine and other agonists of the NMDA glycine site have had mixed results in clinical trials, likely because of their poor oral bioavailability. Perhaps, glycine transporter inhibitors (D'Souza et al, 2011; Umbricht, 2010), which have better bioavailability and are more target specific, might have greater promise.
Consistent with some studies (Kurtz et al, 2009; Silverstein et al, 2009), CRT resulted in improvements in measures of attention/vigilance and verbal working memory. However, unlike other studies (d’Amato et al, 2011; Hodge et al, 2010), CRT did not result in any improvements on reasoning and problem solving, and verbal learning and memory.
There were some site differences on the individual cognitive tasks, but not on the GCI, behavioral ratings, safety, or feasibility. The absence of any consistent pattern in site differences on individual test performance makes it challenging to provide a coherent explanation for site differences. Although admittedly speculative, differences in education, language, learning styles, familiarity, and comfort using computers, transportation, D-serine metabolism, and NMDA receptor function across sites could influence treatment outcomes. These and other site differences pose some challenges to conducting international studies. Future studies will need to attempt to account for these differences especially as clinical trials have become increasingly global.
The rates of improvement on neuropsychological test performance were surprisingly high in all conditions (Supplementary Table 4). In fact, the placebo D-serine–control CRT condition had an improvement rate ranging from 31% (memory encoding) to 54% (for memory retrieval) and an average of 46% across the various neuropsychological domains. The high placebo response that has been reported elsewhere too (Keefe et al, 2008) may also have obscured true treatment effects. It is tempting to speculate to what extent expectancy, payment for study participation, practice effects, or other heretofore unidentified factors contributed to the high placebo-response rate. The high demands related to study participation may have biased toward selecting a less ill sample. In fact, the mean PANSS score of the sample at baseline was low (56.19±16.72). Alternatively, negotiating public transportation and maintaining a budget while commuting to the center twice a week, by itself, may have provided opportunities to exercise cognitive skills and strategies in the real world, and therefore obscured treatment-specific improvements. Such opportunities are known to be vital for functional outcomes (Wykes, 2010) and may have been a confound in this study.
Exploratory Analyses
Exploratory analyses (Supplementary Table S5) revealed that training did not necessarily improve the same domain of cognitive function and, furthermore, training in one cognitive domain was associated with improvements in another domain as has been reported elsewhere (Bell et al, 2009). Verbal Working Memory training was associated with improved Memory Encoding (p=0.05) and cross-modally with Visual Working Memory improvement (p=0.03), but not with Verbal working memory improvement (p=0.77). Attention training had an impact on Verbal Working Memory (p=0.03), although not on Attention (p=0.83). This suggests that researchers and clinicians should be cautious in presuming the specificity of training.
Strengths and Limitations
The strengths of the study include the randomized and placebo-controlled design, the use of tests that overlap with the MATRICS Consensus Battery, low subject attrition, completeness of data, and the novel approach of combining CRT with pharmacotherapy. Finally, plasma levels of D-serine in a random subsample confirmed that D-serine administration (30 mg/kg) resulted in increased plasma D-serine levels significantly. This also provided some evidence of compliance to study medication.
However, the choice of passive TV viewing, rather than active computer games for control CRT, may be a limitation. Relative to more recently developed CRT, the PSSCogRehab (Chen et al, 1997) software may have limitations. Study compensation, although increasing treatment retention, may have inflated placebo response and in doing so, obscured treatment effects. Another limitation of the study is that as some of the outcome measures used had not been rigorously validated for use in India, there are limitations to comparing the results of this study to future studies using similar but validated versions of the outcome measures. Finally, unlike this study, future studies will need to be adequately powered to detect the efficacy of combining D-serine with CRT for cognitive deficits in schizophrenia.
In conclusion, this is the first study demonstrating the feasibility, safety, and tolerability of combination pharmacotherapy and CRT in a multicenter international clinical trial. These preliminary findings provide support for future studies using higher doses of D-serine that have been shown to be efficacious, other pharmacotherapies, or other cognitive-enhancing agents along with the newer cognitive remediation strategies that are individualized and that target basic information processing.
References
Addington D, Addington J, Schissel B (1990). A depression rating scale for schizophrenics. Schizophr Res 3: 247–251.
Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS (1994). Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 116: 143–151.
Barnes TR (1989). A rating scale for drug-induced akathisia. Br J Psychiatry 154: 672–676.
Bell MD, Fiszdon JM, Bryson G (2009). Attention training in schizophrenia: differing responses to similar tasks. J Psychiatr Res 43: 490–496.
Bradley SR, Lameh J, Ohrmund L, Son T, Bajpai A, Nguyen D et al (2010). AC-260584, an orally bioavailable M(1) muscarinic receptor allosteric agonist, improves cognitive performance in an animal model. Neuropharmacology 58: 365–373.
Buchanan RW, Freedm, an R, Javitt DC, Abi-Dargham A, Lieberman JA (2007). Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr Bull 33: 1120–1130.
Chen SH, Thomas JD, Glueckauf RL, Bracy OL (1997). The effectiveness of computer-assisted cognitive rehabilitation for persons with traumatic brain injury. Brain Inj 11: 197–209.
Coyle JT, Tsai G (2004). The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology (Berl) 174: 32–38.
d'Amato T, Bation R, Cochet A, Jalenques I, Galland F, Giraud-Baro E et al (2011). A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Schizophr Research 125: 284–290.
D'Souza DC, Singh N, Elander J, Carbuto M, Pittman B, de Haes JU et al (2012). Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: preliminary evidence. Neuropsychopharmacology 37: 1036–46.
Giustizieri M, Armogida M, Berretta N, Federici M, Piccirilli S, Mercuri NB et al (2008). Differential effect of carbamazepine and oxcarbazepine on excitatory synaptic transmission in rat hippocampus. Synapse 62: 783–789.
Gordon M (1986). Microprocessor-based assessment of attention deficit disorders (ADD). Psychopharmacol Bull 22: 288–290.
Green MF, Kern RS, Braff DL, Mintz J (2000). Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 26: 119–136.
Gustafson EC, Stevens ER, Wolosker H, Miller RF (2007). Endogenous D-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina. J Neurophysiol 98: 122–130.
Guy W (1976). (reprinted 1991)) ECDEU Assessment Manual for Psychopharmacology. U.S. Department of Health, Educaiton and Welfare: Washington DC.
Heinrichs DW, Hanlon TE, Carpenter WT (1984). The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull 10: 388–398.
Heinrichs RW, Zakzanis KK (1998). Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12: 426–445.
Hodge MA, Siciliano D, Withey P, Moss B, Moore G, Judd G et al (2010). A randomized controlled trial of cognitive remediation in schizophrenia. Schizophr Bulletin 36: 419–427.
Homayoun H, Moghaddam B (2010). Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. Eur J Pharmacol 639: 33–39.
Javitt DC (2007). Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol 78: 69–108.
Javitt DC (2009). Glycine transport inhibitors for the treatment of schizophrenia: symptom and disease modification. Curr Opin Drug Discov Devel 12: 468–478.
Javitt DC, Duncan L, Balla A, Sershen H (2005). Inhibition of system A-mediated glycine transport in cortical synaptosomes by therapeutic concentrations of clozapine: implications for mechanisms of action. Mol Psychiatry 10: 275–287.
Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, Balla A, Suckow RF et al (2010). High dose D-serine in the treatment of schizophrenia. Schizophr Res 121: 125–130.
Kapur S, Mamo D (2003). Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 27: 1081–1090.
Kay SR, Opler LA, Lindenmayer JP (1989). The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl 7: 59–67.
Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM et al (2007). Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry 64: 633–647.
Keefe RS, Eesley CE, Poe MP (2005). Defining a cognitive function decrement in schizophrenia. Biol Psychiatry 57: 688–691.
Keefe RS, Malhotra AK, Meltzer HY, Kane JM, Buchanan RW, Murthy A et al (2008). Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology 33: 1217–1228.
Keefe RS, Vinogradov S, Medalia A, Silverstein SM, Bell MD, Dickinson D et al (2011). Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull 37: 1057–1065.
Klingberg S, Wolwer W, Engel C, Wittorf A, Herrlich J, Meisner C et al (2011). Negative symptoms of schizophrenia as primary target of cognitive behavioral therapy: results of the randomized clinical TONES study. Schizophr Bull 37 (Suppl 2): S98–110.
Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R (2003). NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 169: 215–233.
Kurtz MM, Seltzer JC, Fujimoto M, Shagan DS, Wexler BE (2009). Predictors of change in life skills in schizophrenia after cognitive remediation. Schizophr Res. 107: 267–274.
Lee CY, Fu WM, Chen CC, Su MJ, Liou HH (2008). Lamotrigine inhibits postsynaptic AMPA receptor and glutamate release in the dentate gyrus. Epilepsia 49: 888–897.
Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K (1987). The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334: 1–100.
McGurk SR, Mueser KT, Feldman K, Wolfe R, Pascaris A (2007a). Cognitive training for supported employment: 2-3 year outcomes of a randomized controlled trial. Am J Psychiatry 164: 437–441.
McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT (2007b). A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry 164: 1791–1802.
Ninan I, Jardemark KE, Wang RY (2003). Differential effects of atypical and typical antipsychotic drugs on N-methyl-D-aspartate- and electrically evoked responses in the pyramidal cells of the rat medial prefrontal cortex. Synapse 48: 66–79.
O'Donnell CJ, Rogers BN, Bronk BS, Bryce DK, Coe JW, Cook KK et al (2010). Discovery of 4-(5-methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane (CP-810,123), a novel alpha 7 nicotinic acetylcholine receptor agonist for the treatment of cognitive disorders in schizophrenia: synthesis, SAR development, and in vivo efficacy in cognition models. J Med Chem 53: 1222–1237.
Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV (2001a). UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull 27: 235–245.
Patterson TL, Lacro J, McKibbin CL, Moscona S, Hughs T, Jeste DV (2002). Medication management ability assessment: results from a performance-based measure in older outpatients with schizophrenia. J Clin Psychopharmacol 22: 11–19.
Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV (2001b). Social skills performance assessment among older patients with schizophrenia. Schizophr Res 48: 351–360.
Radek RJ, Kohlhaas KL, Rueter LE, Mohler EG (2010). Treating the cognitive deficits of schizophrenia with alpha4beta2 neuronal nicotinic receptor agonists. Curr Pharm Des 16: 309–322.
Rebola N, Srikumar BN, Mulle C (2010). Activity-dependent synaptic plasticity of NMDA receptors. J Physiol 588 (Pt 1): 93–99.
Silverstein SM, Spaulding WD, Menditto AA, Savitz A, Liberman RP, Berten S et al (2009). Attention shaping: a reward-based learning method to enhance skills training outcomes in schizophrenia. Schizophr Bull 35: 222–232.
Simpson GM, Angus JW (1970). A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212: 11–19.
Tsang HW, Leung AY, Chung RC, Bell M, Cheung WM (2010). Review on vocational predictors: a systematic review of predictors of vocational outcomes among individuals with schizophrenia: an update since 1998. Aust N Z J Psychiatry 44: 495–504.
Umbricht D (2010). Glycine Transporter Type 1 (GLYT1) Inhibitor RG1678: Positive Results of the Proof-of-Concept Study for the Treatment of Negative Symptoms in Schizophrenia’. 49th Annual Meeting of the American College of Neuropsychopharmacology Miami Beach, Florida.
Wykes T (2010). Cognitive remediation therapy needs funding. Nature 468: 165–166.
Wykes T, Spaulding WD (2011). Thinking about the future cognitive remediation therapy--what works and could we do better? Schizophr Bull 37 (Suppl 2): S80–90.
Acknowledgements
This study was supported by funding from the Stanley Medical Research Institute (to Deepak C D’Souza), the Donaghue Foundation (to Deepak C D’Souza), a VA Career Award (to Edward Perry), the VA Schizophrenia Center, and the VA Cooperative Studies Program. We thank John H Krystal for his advice in conceptualizing the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Deepak Cyril D’Souza currently receives research grant support administered through Yale University School of Medicine from Astra Zeneca, Abbott Laboratories, Eli Lilly Inc., Organon, Pfizer , and Sanofi, and is a consultant to Bristol Meyers Squibb. Danielle Abi-Saab is currently an employee of Roche Pharmaceuticals and owns stocks with Roche. Her spouse is an employee of Shire and they also own stocks in Shire, Abbott Labs, and Novartis. Chittaranjan Andrade: has received support to conduct clinical trials from Phyto-Pharma, Gufic Ltd, Zandu Pharmaceuticals, Aristo Pharmaceuticals, Cybele Laboratories, Himalaya Drug Company, Natural Remedies, Arya Vaidya Nilayam, Lupin Laboratories, Corcept, Natreon, and Glaxo SmithKline. He has also received lecture fees from Pfizer (payment made directly to a registered charity). He publishes an e-newsletter, which is supported by Sun Pharmaceuticals (payments are made directly to registered charities). He has provided paid consultancy to Astra Zeneca, Wyeth, Sun Pharma, Intas Pharma, and Torrent Pharma (payments either in the form of textbooks or other academic materials or directed to charities). He is also principal investigator and medical monitor for a multicenter, investigator-initiated investigation of the efficacy of Sensoril, funded by Natreon. He authors and receives authorship payments for Critical Readings in Psychiatry series (current publisher, Zydus Neurosciences). The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
D'Souza, D., Radhakrishnan, R., Perry, E. et al. Feasibility, Safety, and Efficacy of the Combination of D-Serine and Computerized Cognitive Retraining in Schizophrenia: An International Collaborative Pilot Study. Neuropsychopharmacol 38, 492–503 (2013). https://doi.org/10.1038/npp.2012.208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2012.208
Keywords
This article is cited by
-
Efficacy and acceptability of psychosocial interventions in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence
Molecular Psychiatry (2023)
-
Cross species review of the physiological role of d-serine in translationally relevant behaviors
Amino Acids (2023)
-
Efficacy of Cognitive Training Program Given to Patients with Schizophrenia Using Computer Tablets: a Preliminary Study
International Journal of Cognitive Therapy (2023)
-
Glutamatergic dysfunction in Schizophrenia
Translational Psychiatry (2022)
-
Evaluation of the Efficacy of BI 425809 Pharmacotherapy in Patients with Schizophrenia Receiving Computerized Cognitive Training: Methodology for a Double-blind, Randomized, Parallel-group Trial
Clinical Drug Investigation (2020)