Abstract
Converging evidence implicates the regulatory neuropeptide Y (NPY) in anxiety- and depression-related behaviors. The present study sought to assess whether there is an association between the magnitude of behavioral responses to stress and patterns of NPY in selected brain areas, and subsequently, whether pharmacological manipulations of NPY levels affect behavior in an animal model of PTSD. Animals were exposed to predator-scent stress for 15 min. Behaviors were assessed with the elevated plus maze and acoustic startle response tests 7 days later. Preset cutoff criteria classified exposed animals according to their individual behavioral responses. NPY protein levels were assessed in specific brain regions 8 days after the exposure. The behavioral effects of NPY agonist, NPY-Y1-receptor antagonist, or placebo administered centrally 1 h post-exposure were evaluated in the same manner. Immunohistochemical technique was used to detect the expression of the NPY, NPY-Y1 receptor, brain-derived neurotrophic factor, and GR 1 day after the behavioral tests. Animals whose behavior was extremely disrupted (EBR) selectively displayed significant downregulation of NPY in the hippocampus, periaqueductal gray, and amygdala, compared with animals whose behavior was minimally (MBR) or partially (PBR) disrupted, and with unexposed controls. One-hour post-exposure treatment with NPY significantly reduced prevalence rates of EBR and reduced trauma-cue freezing responses, compared with vehicle controls. The distinctive pattern of NPY downregulation that correlated with EBR as well as the resounding behavioral effects of pharmacological manipulation of NPY indicates an intimate association between NPY and behavioral responses to stress, and potentially between molecular and psychopathological processes, which underlie the observed changes in behavior. The protective qualities attributed to NPY are supported by the extreme reduction of its expression in animals severely affected by the stressor and imply a role in promoting resilience and/or recovery.
Similar content being viewed by others
INTRODUCTON
Neuropeptide Y (NPY), a 36 amino-acid peptide, is highly conserved among species and widely distributed in the central nervous system (CNS), with high concentration in several limbic and cortical regions (de Quidt and Emson, 1986; Kask et al, 2002). NPY has a role in the regulation of various basic physiological functions, such as food intake (Beck, 2000; Clark et al, 1985; Gehlert, 1999; Kalra et al, 1999), metabolic functions (Krysiak et al, 1999; Small et al, 1997), circadian rhythm (White, 1993), cognition (Flood et al, 1987; Redrobe et al, 1999), neuronal excitability (Colmers and Bleakman, 1994), and addictions and modulation of emotional responses to various stressors (Heilig and Widerlöv, 1995; Mathé et al, 2007). The biological actions of NPY are mediated by the activation of at least five molecularly defined G-coupled receptors family known as the Y1, Y2, Y4, Y5, and Y6 receptor subtypes (Kopp et al, 2002; Michel et al, 1998).
Evidence from behavioral, pharmacological, and genetic studies of the NPY-ergic system in relation to anxiety-related and depression-related behaviors and stress response suggests that the system is involved in stress regulation and coping. The release of NPY is thought to facilitate the containment of negative consequences following exposure to stress and has anxiolytic-like effects (Heilig, 2004). Intracerebroventricular administration of NPY or NPY-Y1-receptor agonists decrease anxiety-related behaviors in the Geller–Seifter conflict test, fear potentiated startle test, and the elevated plus maze (EPM) test, without altering locomotor activity (Britton et al, 1997, 2000; Broqua et al, 1995; Heilig et al, 1989; Primeaux et al, 2005). Intracerebroventricular administration of antisense oligonucleotides for the NPY-Y1 receptor or the non-peptide NPY-Y1-receptor antagonist (Y1RA), BIBP3226, increases anxiety-related behaviors in the EPM test (Kask et al, 1996; Wahlestedt et al, 1993). Moreover, mice deficient in NPY are more anxious than wild type in the EPM (Palmiter et al, 1998), in the open field and the acoustic startle tests (Bannon et al, 2000). Conversely, rats overexpressing NPY in the hippocampus are less anxious (Thorsell et al, 2000). The specific Y1 antagonist BIBO3304 blocks the anxiolytic-like effect of NPY administrated into amygdala in the social interaction test (Sajdyk et al, 1999). In addition, a recent study demonstrated that overexpression of NPY in the amygdala of rats reduced anxiety-like behaviors through Y1 receptor (Primeaux et al, 2005). Taken together, these studies suggest that anxiolytic-like effects of NPY are mediated via Y1 receptors. It seems that NPY, acting via NPY-Y1 receptors, may modulate the noradrenergic and serotonergic systems and influence cortical and limbic functions (Goyal et al, 2009).
In combat veterans with PTSD compared with healthy controls, low baseline and blunted yohimbine-stimulated increases in plasma NPY and negative correlations between baseline NPY, degree of combat exposure, PTSD, and panic attacks, have been reported (Rasmusson et al, 2000). Yehuda et al (2006) reported an association between NPY and resistance to, or recovery from adverse effects of stress; plasma NPY concentrations were higher in trauma-exposed veterans without PTSD compared with veterans with PTSD and in those showing a greater diminution of symptoms. Recently, Sah et al (2009) reported that PTSD patients had significantly lower concentrations of CSF NPY as compared with the normal comparison subjects. Taken together, these studies demonstrate the possible involvement of NPY in the pathophysiology of PTSD, and provide a rationale for studying its role in animal model for PTSD.
The present study sought to assess the relationship between local NPY levels in selected brain areas and magnitude of behavioral change, using an approach to analyze the behavioral response to predator-scent stress (PSS) in an animal model which distinguishes between individuals according to the degree to which their behavior is affected by the stressor (Cohen and Zohar, 2004; Cohen et al, 2003, 2004, 2005).
The first aim of the study was to establish whether single exposure to the PSS results in a long-term effect on the expression of NPY in the AC, PC, amygdala, hippocampus, periaqueductal gray (PAG) regions, employing a bank of recently harvested frozen rat brains from exposed vs unexposed rats stored according to Cutoff Behavioral Criteria (CBC) classification. The second aim was to perform a controlled, prospective trial to examine the effect of an NPY agonist and NPY-Y1-receptor antagonist (BIBO3304), administered 1 h after stress exposure on a number of behavioral, biomolecular, and physiological parameters. Behavioral responses were assessed on the EPM and ASR tests on day 7 and trauma cue triggered freezing responses on day 8. Prevalence rates for extreme behavioral response (EBR), minimal behavioral response (MBR), and partial behavioral response (PBR) individuals in response to PSS were calculated from these data, in comparison with placebo-treated controls and unexposed (treated and untreated) controls. To complement this, local levels of NPY, NPY-Y1, and brain-derived neurotrophic factor (BDNF) expression in the hippocampus were then evaluated. Circulating corticosterone levels served to assess the overall physiological response. Each of these was then analyzed in terms of each class of behavioral response pattern.
The working hypothesis was that early intervention with NPY would reduce the prevalence rate of EBR and increase the prevalence of PBR and/or MBR as compared with placebo-treated, PSS-exposed controls.
MATERIALS AND METHODS
Animals
In all, 231 male Sprague-Dawley rats weighing 200–250 g were habituated to housing conditions for at least 7 days, housed four/cage in a vivarium with stable temperature and a reversed 12-h light/dark cycle, with unlimited access to food and water. Animals were handled once daily. All testing was performed during the dark phase in dim red light conditions.
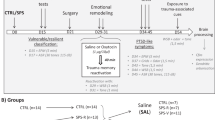
Experimental Design
Three experiments were conducted. In the first, levels of NPY were evaluated in selected areas of harvested brains from animals classified according to CBC's at day 7 post-PSS exposure. In the second, the behavioral effects of NPY agonist and NPY-Y1-receptor antagonist (BIBO3304) microinjected into hippocampus 1 h after PSS exposure were evaluated with the EPM and the ASR tests on day 7. One day later, animals were exposed to a trauma cue (unsoiled cat litter) for 10 min and freezing response was assessed. Local levels of NPY, NPY-Y1, and BDNF in the hippocampus were then evaluated. The last experiment assessed the short-term effect of vehicle (artificial cerebrospinal fluid (ACSF)), NPY, or BIBO3304 microinjection on circulating corticosterone levels.
Predator-Scent Stress
PSS consisted of placing the test animals on well-soiled cat litter (in use by the cat for 2 days, sifted for stools) for 10 min in a closed environment. Control animals were exposed to fresh, unused litter for the same amount of time. The situational reminder consisted of placing animals on fresh, unused cat litter for 10 min.
Behavioral Measurements
Behavioral tests were recorded and analyzed using an EthoVision automated tracking system (Noldus Information Technology, The Netherlands).
The EPM
The maze is a plus-shaped platform with two opposing open and two opposing closed arms (open only toward the central platform and surrounded by 14-cm high opaque walls on three sides; File et al, 1993). Rats were placed on the central platform facing an open arm and allowed to explore the maze for 5 min. Each test was videotaped and subsequently scored by an independent observer. Arm entry was defined as entering an arm with all four paws. Behaviors assessed were time spent (duration) in open and closed arms and on the central platform; number of open and closed arm entries; and total exploration (entries into all arms). Total exploration was calculated as the number of entries into any arm of the maze in order to distinguish between impaired exploratory behavior, exploration limited to closed arms (avoidance), and free exploration.
‘Anxiety index’, an index that integrates the EPM behavioral measures, was calculated as follows:

Anxiety index values range from 0 to 1 where an increase in the index expresses increased anxiety-like behavior (Cohen et al, 2007, 2008b; Mazor et al, 2007).
Acoustic startle response
Startle response was measured using two ventilated startle chambers (SR-LAB system, San Diego Instruments, San Diego, CA). The SR-LAB calibration unit was used routinely to ensure consistent stabilimeter sensitivity between test chambers and over time. Each Plexiglas cylinder rests on a platform inside a sound-proofed, ventilated chamber. Movement inside the tube is detected by a piezoelectric accelerometer below the frame. Sound levels within each test chamber are measured routinely using a sound level meter (Radio Shack) to ensure consistent presentation. Each test session started with a 5-min acclimatization period to background white noise of 68 dB, following by 30 acoustic startle trial stimuli in 6 blocks (110 dB white noise of 40 ms duration with 30 or 45 s inter-trial interval). Behavioral assessment consisted of mean startle amplitude (averaged over all 30 trials) and percent of startle habituation to repeated presentation of the acoustic pulse. Percent habituation—the percent change between the response to the first block of sound stimuli and the last—was calculated as follows:

Contextual Freezing Measurement
Freezing behavior was scored during the situational reminder/cue exposure and was defined as an absence of all movement (except for respiration; Kim et al, 1992). Total cumulative freezing time (total seconds spent freezing during each assessment period) was measured and calculated as a percentage of total time. Freezing behavior was recorded using an overhead video camera and scored for immobility using the recorded images. Both the videotape and the recorded images were scored by a trained observer unaware of the treatment conditions.
RIA Assay of NPY
Tissue preparation
Animals were decapitated with a guillotine in a separate room from the one used for behavioral tests, 24 h after the last behavioral tests (between 1400 and 1430 h). Brains were removed and the anterior cortex (AC), posterior cortex (PC), amygdala (AMY), hippocampus (HIP), and PAG were dissected on ice and frozen at −80°C until used. Each sample was coded and analyses were performed blind to groups.
RIA assay
The radioimmunoassay procedure has been described in previous publications (Stenfors et al, 1989). Briefly, the brain tissues were homogenized, ultrasonicated, and twice extracted in 1 M acetic acid and water. After centrifugation, the supernatants were lyophilized and stored at −28°C until further analysis. The lyophilized samples were reconstituted and diluted in phosphate buffer before analysis by RIA. All samples were run in duplicates. The NPY-LI was assessed using an NPY antibody, a generous gift from M Heilig and R Ekman, that crossreacts 100% with NPY, NPY 2–36, 5% with NPY 5–36, and 0.5% or less with shorter C-terminal NPY fragments. The antibody does not crossreact with pancreatic polypeptide or peptide YY. Samples or standards were pre-incubated with antibody for 48 h at 4°C. After addition of Bolton-Hunter labeled 125I-NPY (Amersham, Bucks, UK) all samples were incubated for additional 24 h. Free and antibody-bound radioligands were separated by addition of sheep anti-rabbit antibody-coated Sepharose suspension (Pharmacia-Upjohn, Uppsala, Sweden). After 30 min incubation at room temperature and centrifugation for 30 min at 1600 g at 4°C, the supernatants were aspirated and discarded. The radioactivity in the pellets was measured in a gamma counter. The lower detection limit was 0.45 pmol/l and the intra-assay coefficient of variation was 5%.
Surgery
Rats were anesthetized with ketamine (60–80 mg/kg, intraperitoneally) and xylazine (5–10 mg/kg, intraperitoneally) and restrained in a stereotactic apparatus (David Kopf, Tujunga, CA). A 26-gauge stainless steel guide cannula (Plastics One, Roanoke, VA) was implanted bilaterally to the dorsal hippocampus (anteroposterior=−3.5 mm, lateral=±2.6 mm, ventral=2.6 mm) (relative to bregma) (Paxinos and Watson, 2005). The cannula was fixed in place with acrylic dental cement and secured by two skull screws. A needle was placed in the guide cannula to prevent clogging. Rats were allowed 7 days to recover before experimental procedures were initiated.
The hippocampus was chosen as a target in this study for several reasons: (1) NPY has been shown to be involved in behavioral functions that depend upon the integrity of the hippocampus (Redrobe et al, 1999). (2) Several reports indicate that mechanisms of action of antidepressants could involve hippocampal NPY (Caberlotto et al, 1998; Mathé et al, 1997). (3) In the hippocampus, in vitro studies have shown that NPY exerts a neuroproliferative effect on neuronal precursors proliferation (Decressac et al, 2010; Howell et al, 2007).
Microinfusions
The stylus was removed, and a 28-gauge injection cannula, extending 1.0 mm from the tip of the guide cannula, was inserted. The injection cannula was connected via PE20 tubing to a Hamilton microsyringe driven by a microinfusion pump (CMA/100; Carnegie Medicine). Microinjections were performed bilaterally with 1 μl per region delivered over 2 min. The injection cannula was left in position for an additional 1 min before withdrawal to minimize dragging of the injected liquid along the injection tract.
Drugs
Both NPY (5 and 10 μg) (Bachem AG—Switzerland) and BIBO3304 (20 μg) ((R)-N-[[4-(aminocarbonylaminomethyl)-phenyl]methyl]-N2-(diphenylacetyl)-argininamide trifluoroacetate) were dissolved in ACSF. Doses were chosen based on previous studies (Pickens et al, 2009).
Histology
At the end of the behavioral tests, 1 μl of India ink was microinjected to identify the cannula placement. After decapitations, the brains were quickly removed, frozen on dry ice, and kept at −20°C. Coronal slices (30 μm) were cut in a cryostat, stained with Nissl stain, and analyzed to verify the microinfusion sites, using diagrams from the atlas by Paxinos and Watson (2005).
Immunofluorescence
Tissue preparation
Twenty-four hours after the behavioral tests, animals were deeply anesthetized (ketamine and xylazine mixture) and perfused transcardially with cold 0.9% physiological saline followed by 4% paraformaldehyde (Sigma-Aldrich) in 0.1 M phosphate buffer (pH 7.4). Brains were quickly removed, post-fixed in the same fixative for 12 h at 4°C, and were cryoprotected overnight in 30% sucrose in 0.1 M phosphate buffer at 4°C. Brains were frozen on dry ice and stored at 80°C. Serial coronal sections (10 μm) at the level of dorsal hippocampus were collected for each animal, using a cryostat (Leica CM 1850) and mounted on coated slides.
Sliced sections were air dried and incubated in frozen methanol (2 min) and in 4% paraformalaldehyde (4 min). After three washes in phosphate buffer saline (PBS) containing Tween-20 (PBS/T) (Sigma-Aldrich), the sections were incubated for 60 min in a blocking solution in PBS (containing normal goat or horse serum) and then overnight at 4°C with the primary antibodies against NPY, BDNF, and GR (1 : 250 each; Abcam). After three washes in PBS/T, sections were incubated in DyLight-488 labeled goat anti-rabbit IgG or Dylight-594 goat anti-mouse IgG (1 : 250; KPL, MD) in PBS containing 2% normal goat or house serum for 2 h. Sections were washed, mounted with mounting medium (Vectrastain Vector Laboratories). Control staining was performed in the absence of the primary antibodies. Additionally, secondary fluorescent labels were swapped to check crossreactivity and sections were incubated without any primary antibodies to check for any non-specific binding of the secondary antibodies.
Quantification
A computer-assisted image analysis system (Leica Application Suite V3.6, Leica, Germany) was used for quantitative analysis of the immunostaining and × 50 objective lens were employed to assess the number of NPY, BDNF, and GR-IR-positive cells in the hippocampus, divided into three (counted separately) areas: CA1 subfield, CA3 subfield, and dentate gyrus (DG). The regions of interest were outlined and computer-aided estimation was used to calculate the number of NPY-IR, BDNF-IR, and GR-IR cells in the pyramidal layer of CA1 and CA3, and in the granular layer of DG. Seven representative sections of the hippocampus were chosen (between Bregma −2.30 and Bregma −3.60) from each animal, from each group (Paxinos and Watson, 2005). The sections were analyzed by two observers blinded to the treatment protocol. Standard technique was used to estimate the number of NPY, BDNF, and GR-IR cells profiles per unit area for each investigated hippocampal structure.
Blood sampling
Animals were decapitated with a guillotine. Care was taken to minimize situational stress: the area was thoroughly cleaned between each kill and bodies removed. Trunk blood was collected, left at room temperature for 1 h and then centrifuged (1000 g for 10 min at 4°C). Serum (∼1 ml from each rat) was collected and stored at −70°C.
Measurement of serum corticosterone
CORT was measured with a DSL-10-81000 ELISA kit according to the instructions of the manufacturer (Diagnostic Systems Laboratories, Webster, TX) by a person blind to experimental procedures. The sensitivity of the corticosterone assay is 12.5 μg/l. Within-assay variation is <10% and between-assay variation is <15% at 100 μg/l. All samples were measured in duplicate.
Statistical Analyses
For brain NPY levels and for serum corticosterone levels, the statistical analyses were performed using one-way ANOVA. For the behavioral and immunofluorescence results, the statistical analyses were performed using two-way ANOVA, in which PSS exposure (unexposed vs PSS exposure) and treatment (ACSF vs NPY (5 and 10 μg) vs Y1RA) were factors. Post hoc Bonferroni test examined differences between individual groups. The prevalence of affected rats as a function of rat group was tested using cross-tabulation and non-parametric χ2 tests.
The CBC model
The classification of individuals according to the degree to which their individual behavior is affected by a stressor is based on the premise that in the natural environment, such extremely compromised behavior in response to the priming trigger may compromise behaviors essential for survival, and is thus inadequate and maladaptive, representing a pathological degree of response (Cohen et al, 2011).
RESULTS
Since NPY is involved in the control of food intake, we measured body weight in all rats during all days of treatment. No differences in body weight were observed between the groups.
NPY Levels at Day 7 Post-PSS Exposure
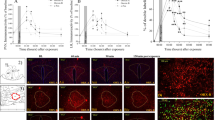
In the PC (Figure 1b), amygdala (Figure 1c), hippocampus (Figure 1d), and PAG (Figure 1e) areas, there were significant differences among groups (one-way ANOVA: F(3, 45)=4.65, p<0.007; F(3, 45)=2.9, p<0.05; F(3, 45)=11.1, p<0.0001; and F(3, 45)=9.5, p<0.000, respectively). Post hoc Bonferroni test confirmed that EBR group exhibited significantly lower NPY protein levels in the PC, amygdala, hippocampus, and PAG as compared with controls (p<0.005, p<0.015, p<0.0001, and p<0.0001, respectively). In the amygdala and PAG areas, the EBR group exhibited significantly lower NPY levels as compared with the MBR animals (p<0.02 and p<0.025, respectively). In the PC, hippocampus, and PAG areas, the PBR also exhibited significantly lower NPY protein levels as compared with unexposed control group (p<0.05, p<0.0002, and p<0.0085, respectively). In the hippocampus, the MBR group exhibited significantly lower NPY protein levels as compared with unexposed control group (p<0.006). In the AC region (Figure 1a), no significant differences were found between groups.
NPY levels at day 7 post-PSS exposure. RIA analysis of NPY protein levels in the hippocampal anterior cortex (a), posterior cortex (b), amygdala (c), hippocampus (d), and periaqueductal gray (PAG) (e) between unexposed group (N=10), EBR (11), PBR (15), and MBR (13) groups. EBR animals exhibited significantly lower NPY levels in posterior cortex, amygdala, hippocampus, and PAG areas as compared with unexposed animals. EBR, extreme behavioral response; MBR, minimal behavioral response; PBR, partial behavioral response; PSS, predator-scent stress; NPY, neuropeptide Y; Y1RA, neuropeptide Y-receptor 1 antagonist. All data represent group mean±SEM.
The Behavioral Effects of Administration of NPY and BIBO3304 1 h After PSS Exposure
Elevated plus maze
Two-way ANOVA revealed a significant PSS exposure and treatment effects in terms of time spent in open arms (F(1, 79)=39.5, p<0.0001 and F(3, 79)=4.8, p<0.004, respectively; Figure 2a). No effects were observed for PSS–treatment interaction. In terms of open arms entries, there was a significant effect of PSS exposure (F(1, 79)=34.6, p<0.0001; Figure 2b). No effects were observed for treatment (F(3, 79)=2.62, p=0.056) or exposure–treatment interaction (F(3, 79)=7.2, p=0.082). In total activity on the maze, there were a significant PSS exposure, treatment, and PSS–treatment interaction effects in terms of time spent in open arms (F(1, 79)=18.7, p<0.0001, (3,79)=9.8, p<0.0001 and F(3, 79)=5.4, p<0.0002, respectively; Figure 2c). For the anxiety index, two-way ANOVA revealed a significant effects of PSS exposure (F(1, 79)=42.7, p<0.0001) and for treatment (F(3, 79)=3.9, p<0.012; Figure 2d). No effect was observed for exposure–treatment interaction. Bonferroni test confirmed that exposed group treated with ACSF, NPY 5 μg, or BIBO3304 elicited a significant decrease in overall time spent in open arms (p<0.03, p<0.0001, and p<0.0001, respectively) and a significantly increased anxiety index (p<0.03, p<0.0003, and p<0.0001) as compared with unexposed controls. No differences were observed in overall time spent in the open arms of the maze or in the anxiety index between PSS-exposed animals treated with NPY 10 μg and the unexposed NPY controls. Exposed group treated with NPY 10 μg spent significantly more time in the open arms of the maze (p<0.0004, p<0.04, and p<0.05, respectively) and exhibited lower anxiety index (p<0.02, p<0.04, and p<0.05, respectively) than exposed animals treated with BIBO3304, NPY 5 μg, or ACSF.
Long-term behavioral effects of administration of NPY and BIBO3304 1 h after PSS exposure. Top line: The behavioral procedure used for the unexposed and PSS-exposed rats. Vertical arrow represents hippocampus microinjection (NPY agonist (5 and 10 mg/kg), NPY-Y1-receptor antagonist (20 μg), or ACSF). (a) Time spent in the open arms (min), (b) the number of entries to the open arms of the maze, (c) total activity on the maze, and (d) anxiety index were evaluated between unexposed animals treated with ACSF (N=10), NPY agonist 5 μg (N=10), NPY agonist 10 μg (N=10), and Y1RA (N=14) and between PSS-exposed animals treated with ACSF (N=10), NPY agonist 5 μg (N=10), NPY agonist 10 μg (N=10), and Y1RA (N=12). A single 10-min exposure to PSS followed by administration of NPY (10 mg/kg) significantly increased the time spent in the open arms and increased anxiety index as compared with ACSF treatment. On the other hand, administration of NPY-Y1-receptor antagonist 1 h after PSS exposure significantly decreased the time spent in the open arms, the number of entries to the open arms of the maze, and total exploration on the maze and decreased anxiety index as compared vehicle treatment or treatment with NPY (10 mg/kg). PSS, predator-scent stress; NPY, neuropeptide Y; Y1RA, neuropeptide Y-receptor 1 antagonist; ACSF, artificial cerebrospinal fluid. All data represent group mean±SEM.
ASR
Two-way ANOVA revealed a significant effect for PSS exposure (F(1, 79)=49.7, p<0.0001), a treatment effect (F(3, 79)=5.3, p<0.035), and an exposure–treatment interaction effect (F(3, 79)=6.8, p<0.0007) (Figure 3a). Bonferroni test confirmed that exposed groups treated with ACSF or BIBO3304 significantly increased mean startle amplitude, as compared with these unexposed controls (p<0.0001 for both). No differences were observed in mean startle amplitude between PSS-exposed animals treated with NPY (both doses) and the unexposed controls. Animals treated with NPY (both doses) exhibited significantly lower startle amplitude as compared with exposed animals treated with ACSF (p<0.0001) or BIBO3304 (p<0.00001).
Long-term effects of administration of NPY agonist and BIBO3304 1 h after PSS exposure. Top line: The behavioral procedure used for the unexposed and PSS-exposed rats. Vertical arrow represents hippocampus microinjection (NPY agonist (5 and 10 mg/kg), NPY-Y1-receptor antagonist (20 μg), or ACSF). (a) Startle amplitude and (b) startle habituation were evaluated between unexposed animals treated with ACSF (N=10), NPY agonist 5 μg (N=10), NPY agonist 10 μg (N=10), and Y1RA (N=14) and between PSS-exposed animals treated with ACSF (N=10), NPY agonist 5 μg (N=10), NPY agonist 10 μg (N=10), and Y1RA (N=12). Exposed groups treated with ACSF or BIBO3304 exhibited significantly higher mean startle amplitude and a significant deficit in startle habituation, as compared with their unexposed controls. No differences were observed in mean startle amplitude or startle habituation between PSS-exposed animals treated with NPY (both doses) and the unexposed controls. PSS, predator-scent stress; NPY, neuropeptide Y; Y1RA, neuropeptide Y-receptor 1 antagonist; ACSF, artificial cerebrospinal fluid. All data represent group mean±SEM.
Startle habituation
Two-way ANOVA revealed a significant effect for PSS exposure (F(1, 79)=19.0, p<0.0008) and for treatment effect (F(3, 79)=3.7, p<0.02) (Figure 3b). No effect was observed for exposure–treatment interaction. Bonferroni test confirmed that PSS exposure caused a significant deficit in habituation in exposed animals treated with ACSF or BIBO3304 compared with unexposed control groups (p<0.02 and p<0.0005, respectively) and to exposed animals treated with NPY 5 μg (p<0.008 and p<0.02, respectively) or NPY 10 μg (p<0.02 and p<0.05, respectively). No differences were observed in startle habituation between PSS-exposed animals treated with NPY (at both doses) and unexposed ACSF or NPY controls.
Relative prevalence rates according to CBC's
There were significant differences in the prevalence rates of individuals displaying EBR among groups (Pearson χ2=37.1, df=7, p<0.00001). As shown in Figure 4a, the prevalence of EBR individuals among PSS-exposed rats injected with ACSF was 40.0% of the total population and differed significantly from the unexposed group (χ2=5.0, p<0.03) and from the exposed group treated with NPY 10 μg (χ2=5.0, p<0.03), in which there were no EBR individuals. The prevalence of EBR individuals among PSS-exposed rats injected with BIBO3304 was 66.7% of the total population and differed significantly from the unexposed group (χ2=10.2, p<0.009) and from the exposed group treated with NPY (both doses). There were significant differences in the prevalence rates of individuals displaying MBR among groups (Pearson χ2=28.0, df=7, p<0.00025; Figure 4b). The prevalence of MBR among the PSS-exposed rats injected with NPY at 10 μg was 40.0%, and differed significantly from exposed animals treated with ACSF (χ2=2.5, p<0.03) or from exposed animals treated with BIBO3304 (χ2=5.9, p<0.02). There were no significant differences in the prevalence of PBR among groups (Figure 4c).
Long-term effects of administration of NPY agonist and BIBO3304 1 h after PSS exposure on the prevalence of EBR, PBR, and MBR individuals. Top line: The behavioral procedure used for the unexposed and PSS-exposed rats. Vertical arrow represents hippocampus microinjection (NPY agonist (5 and 10 mg/kg), NPY-Y1-receptor antagonist (20 μg), or ACSF). (a) Prevalence of EBR rats, (b) prevalence of MBR rats, and (c) prevalence of PBR rats were estimated between unexposed animals treated with ACSF (N=10), NPY agonist 5 μg (N=10), NPY agonist 10 μg (N=10), and Y1RA (N=14) and between PSS-exposed animals treated with ACSF (N=10), NPY agonist 5 μg (N=10), NPY agonist 10 μg (N=10), and Y1RA (N=12). Early treatment with NPY (10 mg/kg) reduced the prevalence of PTSD-like behavioral responses (EBR) relative to ACSF or NPY-Y1-receptor antagonist treatments. PSS, predator-scent stress; NPY, neuropeptide Y; Y1RA, neuropeptide Y-receptor 1 antagonist; ACSF, artificial cerebrospinal fluid.
Effect of Cue Exposure on Freezing Behavior at Day 8
Two-way ANOVA revealed a significant effect for PSS exposure (F(1, 79)=16.3, p<0.00015) and a treatment effect (F(3, 79)=7.0, p<0.0003) (Figure 5). No effect was observed for exposure–treatment interaction (F(3, 79)=2.3, p=0.08). Bonferroni test confirmed that exposed rats treated with saline displayed significantly more immobility than unexposed controls (p<0.00015) and exposed rats treated with NPY (5 and 10 μg; p<0.02 and p<0.0002, respectively). No differences were observed between PSS-exposed animals treated with NPY (at 2 doses) and their unexposed controls.
Long-term effects of administration of NPY agonist and BIBO3304 1 h after PSS exposure on freezing behavior. Top line: The behavioral procedure used for the unexposed and PSS-exposed rats. Vertical arrow represents hippocampus microinjection (NPY agonist (5 and 10 mg/kg), NPY-Y1-receptor antagonist (20 μg), or ACSF). Freezing response was assessed between unexposed animals treated with ACSF (N=10), NPY agonist 5 μg (N=10), NPY agonist 10 μg (N=10), and Y1RA (N=14) and between PSS-exposed animals treated with ACSF (N=10), NPY agonist 5 μg (N=10), NPY agonist 10 μg (N=10), and Y1RA (N=12). Exposed animals treated with NPY (10 mg/kg) displayed significantly less immobility than SCDF or NPY-Y1-receptor antagonist-treated exposed rats. CA1, cornu ammonis 1; PSS, predator-scent stress; NPY, neuropeptide Y; Y1RA, neuropeptide Y-receptor 1 antagonist; ACSF, artificial cerebrospinal fluid. All data represent group mean±SEM.
NPY and NPY-Y1 IR Expression at Day 8 Post-PSS Exposure
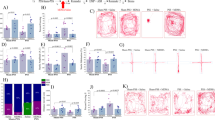
We employed the double immunofluorescence protocol to examine colocalization of NPY and NPY-Y1 IR expression in the hippocampus subregions. The majority of NPY-immunoreactive cells in the hippocampus co-expressed NPY-Y1. However, some NPY-positive cells were devoid of NPY-Y1 immunoreactivity (Figure 6). These results suggest that NPY is co-expressed in the majority of NPY-Y1-positive cells in the hippocampus.
Effect of early post-stressor intervention with NPY agonist and BIBO3304 on NPY and NPY-Y1 immunoreactivity. The quantitative analysis of NPY (a–c) and NPY-Y1 (d–f) immunostaining in the CA1, CA3, and DG hippocampus subregion of unexposed rats treated with ACSF (N=7), NPY agonist (N=7), and BIBO3304 (N=6) and exposed rats treated with ACSF (N=6) NPY agonist (N=7), and BIBO3304 (N=7). (g) Representative photographs of NPY and NPY-Y1 immunoreactivity in the hippocampus subregions. Photographs were acquired at × 50 magnification. Scale bar, 50 μm. The cells in red were NPY positive and in green were NPY-Y1 and in yellow were merge. Administration of NPY 1 h post-exposure significantly increased expression of NPY and NPY-Y1 receptor as compared with exposed animals treated with vehicle. CA1, cornu ammonis 1; PSS, predator-scent stress; NPY, neuropeptide Y; Y1RA, neuropeptide Y-receptor 1 antagonist; ACSF, artificial cerebrospinal fluid. All data represent group mean±SEM.
NPY-IR Expression
In the CA1 subregion, two-way ANOVA revealed a significant effect for PSS exposure (F(1, 34)=25.0, p<0.0001) and a treatment effect (F(2, 34)=10.2, p<0.0004) (Figure 6a). No effect was observed for exposure–treatment interaction. In the DG, two-way ANOVA revealed a significant effect for PSS (F(1, 34)=93.0, p<0.0001), a treatment effect (F(2, 34)=24.6, p<0.0001), and an exposure–treatment interaction (F(2, 34)=15.2, p<0.0001) (Figure 6c). Bonferroni test confirmed that in the CA1 and DG areas, PSS exposure decreased expression of NPY in animals treated with ACSF (p<0.0015 and p<0.0001, respectively) or animals treated with BIBO3304 (p<0.0007 and p<0.0001, respectively) as compared with their unexposed controls. Administration of NPY 1 h post-exposure significantly increased DG NPY-IR expression as compared with exposed animals treated with ACSF (p<0.0001) or animals treated with BIBO3304 (p<0.0001). In the CA1 area, administration of NPY 1 h post-exposure significantly increased NPY-IR expression as compared with BIBO3304 (p<0.0001). In the CA3 subregion, no significant differences were found between groups. In the CA3 subregion, no significant differences were found between groups (Figure 6b).
NPY-Y1 IR Expression
In the hippocampal CA1, two-way ANOVA revealed a significant effect for PSS (F(1, 34)=31.9, p<0.0001), a treatment effect (F(2, 34)=8.8, p<0.0004), and an exposure–treatment interaction (F(2, 34)=3.65, p<0.04) (Figure 6d). In the DG, two-way ANOVA revealed a significant effect for PSS (F(1, 34)=90.4, p<0.0001), a treatment effect (F(2, 34)=28.9, p<0.0001), and an exposure–treatment interaction (F(2, 34)=17.7, p<0.0001) (Figure 6f). Bonferroni test confirmed that in the CA1 and DG areas, PSS exposure decreased expression of NPY-Y1 in animals treated with ACSF (p<0.0003) or animals treated with BIBO3304 (p<0.0001) as compared with their unexposed controls. Administration of NPY post-exposure significantly increased DG NPY-Y1 IR expression as compared with exposed animals treated with ACSF (p<0.0001) or animals treated with BIBO3304 (p<0.0001). In the CA1 area, administration of NPY post-exposure significantly increased NPY-IR expression as compared with BIBO3304 (p<0.0001). In the CA3 subregion, no significant differences were found between groups (Figure 6e).
BDNF-IR Expression at Day 8 Post-PSS Exposure
In the hippocampal CA1, two-way ANOVA revealed a significant effect for PSS (F(1, 34)=22.9, p<0.0001) and a treatment effect (F(2, 34)=12.4, p<0.00015) (Figure 7a). No effect was observed for exposure–treatment interaction. In contrast, in the CA3, no effect was observed for PSS or exposure–treatment interaction, but a significant effect for treatment effect was found (F(2, 34)=47.4, p<0.0001; Figure 7b). In the DG, in similarity to the CA1, there was a significant effect for PSS (F(1, 34)=6.4, p<0.02), a treatment effect (F(2, 34)=15.4, p<0.0001), and an exposure–treatment interaction (F(2, 34)=1.2, p<0.003) (Figure 7c). Bonferroni test confirmed that in the CA1 and DG areas, PSS exposure decreased expression of BDNF in animals treated with ACSF (p<0.006 and p<0.035, respectively) and animals treated with BIBO3304 (p<0.007 and p<0.0002, respectively) as compared with their unexposed controls. Administration of NPY 1 h post-PSS significantly increased CA3 and DG BDNF-IR expression as compared with exposed animals treated with ACSF (p<0.0001 and p<0.004, respectively) or with BIBO3304 (p<0.0001 for both). In the CA1 area, administration of NPY 1 h post-PSS exposure significantly increased BDNF-IR expression as compared with BIBO3304 (p<0.005).
Effect of early post-stressor intervention with NPY agonist and BIBO3304 on BDNF immunoreactivity in the hippocampus subregions: The quantitative analysis of BDNF immunostaining in the hippocampus subregions CA1 (a), CA3 (b), and DG (c) of unexposed rats treated with ACSF (N=7), NPY agonist (N=7), and BIBO3304 (N=6) and exposed rats treated with ACSF (N=6) NPY agonist (N=7), and BIBO3304 (N=7). Administration of NPY 1 h post-exposure significantly increased expression of BDNF in the CA3 and DG areas as compared with exposed animals treated with vehicle and animals treated with NPY-Y1-recptor antagonist. DG, dentate gyrus; CA1, cornu ammonis 1; PSS, predator-scent stress; NPY, neuropeptide Y; Y1RA, neuropeptide Y-receptor 1 antagonist; BDNF, brain-derived neurotrophic factor; ACSF, artificial cerebrospinal fluid. All data represent group mean±SEM.
Effects of vehicle/NPY and BIBO3304 microinfusion on circulating corticosterone levels
To examine the effect of NPY on the circulating corticosterone levels, vehicle, NPY, and BIBOP3304 were microinjected into the dorsal hippocampus and killed 15, 30, and 60 min after, for evaluation of corticosterone (Figure 8). Rats treated with NPY displayed significantly higher serum corticosterone levels 15, 30, and 60 min after microinjection as compared with vehicle or BIBO3304 microinjections or with baseline levels (15 min: F(2, 9)=7.1, p<0.001; 30 min: F(2, 9)=5.5, p<0.03; and 60 min: F(2, 9)=27.6, p<0.0002, respectively—Bonferroni post hoc: p<0.001 for all).
Effects of NPY, BIBO3304, and vehicle microinfusion on circulating corticosterone levels. Top line: The behavioral procedure used for the unexposed and PSS-exposed rats. Vertical arrow represents hippocampus microinjection (NPY agonist (5 and 10 mg/kg), NPY-Y1-receptor antagonist (20 μg), or ACSF). Circulating corticosterone levels followed by hippocampus microinjection of NPY, BIBOP3304, or vehicle. Microinjection of NPY (10 μg) significantly increased serum corticosterone levels after 15 min as compared with vehicle or BIBO3304 microinjections or with baseline levels. NPY, neuropeptide Y; Y1RA, neuropeptide Y-receptor 1 antagonist; *p<0.05 vs baseline. #p<0.05 vs Vehicle and BIBO3304. All data represent group mean±SEM.
DISCUSSION
The most significant findings of this study are (1) expression of NPY was markedly decreased in selected brain regions in animals showing behavioral changes in this model of PTSD, (2) following blockade of the NPY-Y1-receptor PSS had extreme effects, indicating the crucial protective role of endogenous NPY, and (3) centrally administered NPY elevated endogenous NPY and rescued the behavioral effects of PSS. There was a striking association between the degree of behavioral disruption and the pattern of NPY expression. Animals whose behavior was extremely disrupted (EBR) selectively displayed significant downregulation of NPY in the PAG, hippocampus, and amygdala compared with unexposed controls. Manipulation of NPY levels had a significant impact on patterns of behavioral and neurobiological responses to the PSS paradigm. Administration of NPY into hippocampus 1 h post-exposure significantly reduced behavioral disruption and was associated with upregulation of NPY, NPY-Y1 receptor, and BDNF expression. This finding is of interest as it raises the possibility that exogenous NPY can enhance the expression of endogenous NPY. In view of the activity of peptidases in the brain as well as no documentation for existence of active uptake/diffusion of NPY into cells it is unlikely that the observed increase of number of immunohistochemistry-positive NPY cells 8 days following administration represents the injected NPY. In contrast, it stands to reason that the increased NPY immunoreactivity represents increased NPY expression, considering that NPY expression can be enhanced by epigenetic mechanisms, which is in line with the general knowledge of epigenetic mechanisms modulating expression of a variety of genes. Further, in opposition to but consistently with the effects of injected NPY, NPY-Y1-receptor antagonist injected 1 h after PSS exposure was associated with an extreme degree of behavioral disruption in the EPM and ASR tests, reflected by a pronounced increase in prevalence rates of EBR and in trauma-cue freezing responses, relative to ACSF or NPY treatment. This result demonstrates the crucial stress protective effect of endogenous NPY that is exerted via NPY-Y1 receptor. Taken together, these findings indicate that NPY-ergic system has an active role in the neurobiological response to PSS and that the response is mediated via the NPY Y1 receptor.
The initial stage of the study examining local brain levels of NPY in stress-exposed animals revealed that 8 days after exposure NPY levels in the PC amygdala hippocampus and PAG were downregulated in animals whose behavior was severely affected by the stressor (EBR). In light of the neuroprotective and neuronal growth-promoting effects attributed to NPY, the observed decrease in NPY expression characterizing the severely affected animals suggests that NPY may actively contribute to recovery and/or resilience to stress. These findings imply a possible association between the molecular findings and psychopathological processes which result in altered behavior. It is unclear whether they equally have a causal role bringing about the disordered stress response and/or represent markers thereof. The results are in line with our hypothesis and previous findings that vicissitudes of NPY have an important role in regulation of anxiety and depression (Domschke et al, 2010; Heilig, 2004; Mathé, 1999; Mathé et al, 1996; Neumann et al, 2011; Redrobe et al, 1999; Sajdyk et al, 2008).
In line with such a hypothesis, a single dose of NPY microinfused into the dorsal hippocampus 1 h after exposure resulted in a significant reduction of behaviors representing anxiety and avoidance responses. Relative to untreated and vehicle-treated controls, a single 10 μg of NPY administration reduced prevalence rates of extreme PTSD-like behavioral response patterns to nil, with concomitant increases in prevalence rates of minimal response pattern as compared with vehicle treatment—ie, a significant overall shift toward less extreme behavioral disruption ensuing from traumatic stress. The NPY-treated group also demonstrated markedly less extreme freezing responses to the trauma cue (17.0% of time freezing) than the exposed vehicle-control group (37.0% of time freezing). In contrast, brief treatment with NPY-Y1-receptor antagonist, initiated 1 h after stress exposure, was associated with significantly poorer long-term outcome than exposed vehicle control or even unexposed vehicle control. NPY-Y1-receptor antagonist treatment was associated with a far greater degree of behavioral disruption in the EPM and ASR tests, reflected by a pronounced increase in prevalence rates of EBR and in trauma-cue freezing responses, relative to exposure vehicle group. This result reinforces the role of deregulated NPY transmission in anxiety and depression, points to the important protective role of endogenous NPY, and raises the prospect of NPY-Y1-receptor agonists as potential treatments for affective disorders and anxiety.
The anxiolytic-like effect of NPY 10 μg microinjected 1 h after PSS exposure was accompanied by a significant upregulation of NPY, NPY-Y1, and BDNF-IR cells compared with exposed animals treated with vehicle. In contrast, microinjection of NPY-receptor antagonist was accompanied by a significant downregulation of NPY, NPY-Y1, and BDNF in the hippocampus. Therefore, it is a reasonable assumption that NPY-Y1 receptors in the hippocampus are involved in the anxiolytic effects of NPY (Heilig, 2004; Karlsson et al, 2008). Moreover, there was a strong overlap between the widely distributed NPY and NPY-Y1 IR cells in the hippocampus, suggesting that in most cases there are NPY-Y1 receptors fairly close to the NPY-containing (releasing) nerve endings.
Previously, it has been suggested that BDNF may serve as a regulator of the functional and morphological expression of the NPY neuron (Barnea and Roberts, 2001); for instance, Jones et al (1994) reported that the number of NPY neurons was significantly lower in the cortex and hippocampus of mice homozygous for a BDNF null mutation than in control mice. BDNF, administered in vitro (Nawa et al, 1993) or in vivo (Nawa et al, 1994), induces an increase in NPY mRNA and peptide content. Xapelli et al (2008) reported that the expression of NPY in the hippocampus is under the control of BDNF receptor activity. Neurotrophins, and particularly BDNF, are known to modulate many aspects of neuronal plasticity (Shieh and Ghosh, 1999; Thoenen, 2000) and the selection of functional neuronal connections in the CNS (Huang and Reichardt, 2001; Mamounas et al, 2000; Poo, 2001). Thus, the increased expression of BDNF following NPY microinjection may increase synaptic plasticity and stabilization of synaptic connectivity, leading to resilience to psychopathology. These responses could modulate neuronal plasticity and excitability and may serve as a cellular mechanism for neuroprotection and neuroplasticity. In line with our results, Croce et al (2011) have reported that in SH-SY5Y neuroblastoma cells administration of NPY increases the survival and counteracts the toxic effect of β-amyloid. In addition, NPY increased BDNF and NGF protein levels in these cells. In contrast, Gelfo et al (2011) reported that a 3-day NPY treatment decreased BDNF and increased NGF expression in the hypothalamus. Since the mechanism of action of NPY on neurotrophins is not known, it is possible that NPY, that acts at both pre- and post-synaptic receptors, may have different effects on BDNF expression depending on the type of external stimuli BDNF is capable of stimulating its own release, possibly allowing sustained, regenerative signaling at synaptic sites (Bramham and Messaoudi, 2005).
In order to assess the interaction between NPY and the HPA axis, levels of circulating corticosterone were measured in response to vehicle, NPY, or NPY-Y1-receptor antagonist microinjections. The NPY-treated rats displayed significantly higher circulating corticosterone levels than vehicle controls or NPY-Y1-receptor antagonist treatment. We hypothesize that exogenous NPY effects on anxiety-like behavior might be mediated through an NPY-induced alteration in glucocorticoid release from the adrenal gland. This pattern conforms to an adaptive HPA-axis stress–response curve, suggesting that NPY-induced secretion of corticosterone following stress exposure may be associated with recovery processes and/or indicate resilience. This hypothesis is supported by previous studies suggesting that a blunted HPA-axis response to stress may have a role in the susceptibility to experimentally induced PTSD-like behavioral changes and that these effects may be reversed by pre-exposure administration of corticosterone (Cohen et al, 2006). Additionally, treatment with high-dose corticosterone 1 h after stressful exposure reduces the prevalence rate of extreme behavioral disruption 30 days later (Cohen et al, 2008a). These results confirm the bulk of evidence indicating that NPY stimulates the activity of the central branch of the HPA axis, thereby enhancing glucocorticoid secretion from the adrenal cortex during prolonged maternal absence (Schmidt et al, 2008). Furthermore, a recent study in a rat model of depression has evidenced increased BDNF levels in the hypothalamus together with increased systemic levels of adrenocorticotropin hormone and corticotropin-releasing hormone, suggesting a possible role of BDNF in HPA-axis hyper-activation (Naert et al, 2011). Taken together, exogenous NPY effects on anxiety-like behavior might be mediated through an NPY-induced alteration in glucocorticoid release from the adrenal gland. The glucocorticoids not only exert a negative feedback effect on the HPA axis, but also activate production of endogenous neurotrophic (BDNF) signaling, concomitant with the induction of endogenous NPY. Endogenous BDNF and NPY in the hippocampus may provide intrinsic cortical neurons with more neurotrophic support and thus enable the organism to adjust to the (altered) prevailing conditions and re-establish homeostasis. In view of these and previous data, we hypothesize that the NPY-ergic system, in modulating stress-related responses via its redundancy and overlapping functionality, provides regulatory mechanisms to modulate stress response.
Conclusions
This study shows that NPY has a role in the stress–response cascade, interacting with other systems including the HPA axis to mediate processes involved in stress-related behavioral responses, memory consolidation, recovery, and resilience. Significantly, it (1) contributes additional evidence to the experimental results both that NPY has a central role in conditions of ‘disturbed emotionality’, such as depression (Heilig, 2004; Heilig et al, 2004; Husum et al, 2006; Jiménez-Vasquez et al, 2007; Mathé et al, 2007; Nikisch et al, 2005), anxiety (Thorsell et al, 2000; Neumann et al, 2011), ethanol preference (Ehlers et al, 1998), chronic stress (Sergeyev et al, 2005), and early life adverse events (Jiménez-Vasquez et al, 2001; Husum and Mathé, 2002) and (2) reinforces the proposition that NPY and the NPY Y1-receptor agonists are potential novel therapeutic targets for the stress-related disorders, of importance in light of the insufficient efficacy of current drugs targeting monoamines.
References
Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B et al (2000). Behavioral characterization of neuropeptide Y knockout mice. Brain Res 868: 79–87.
Barnea A, Roberts J (2001). Induction of functional and morphological expression of neuropeptide Y (NPY) in cortical cultures by brain-derived neurotrophic factor (BDNF): evidence for a requirement for extracellular-regulated kinase (ERK)-dependent and ERK-independent mechanisms. Brain Res 919: 57–69.
Beck B (2000). Neuropeptides and obesity. Nutrition 16: 916–923.
Bramham C, Messaoudi E (2005). BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76: 99–125.
Britton K, Akwa Y, Spina M, Koob G (2000). Neuropeptide Y blocks anxiogenic-like behavioral action of corticotropin-releasing factor in an operant conflict test and elevated plus maze. Peptides 21: 37–44.
Britton K, Southerland S, Van Uden E, Kirby D, Rivier J, Koob G (1997). Anxiolytic activity of NPY receptor agonists in the conflict test. Psychopharmacology 1325: 6–13.
Broqua P, Wettstein J, Rocher M, Gauthier-Martin B, Junien J (1995). Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear potentiated startle procedures. Behav Pharmacol 6: 215–222.
Caberlotto L, Fuxe K, Overstreet D, Gerrard P, Hurd Y (1998). Alterations in neuropeptide Y, Y1 receptor mRNA expression in brains from an animal model of depression: Region specific adaptation after fluoxetine treatment. Mol Brain Res 59: 58–65.
Clark JT, Kalra PS, Kalra SP (1985). Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology 117: 2435–2442.
Cohen H, Kaplan Z, Matar MA, Loewenthal U, Zohar J, Richter-Levin G (2008a). Long-lasting behavioral effects of juvenile trauma in an animal model of PTSD associated with a failure of the autonomic nervous system to recover. Eur Neuropsychopharmacol 17: 464–477.
Cohen H, Kozlovsky N, Matar MA, Zohar J, Kaplan Z (2011). The characteristic long-term upregulation of hippocampal NF-kappaB complex in PTSD-like behavioral stress response is normalized by high-dose corticosterone and pyrrolidine dithiocarbamate administered immediately after exposure. Neuropsychopharmacology 36: 2286–2302.
Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J (2008b). Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol Psychiatry 64: 708–717.
Cohen H, Zohar J (2004). An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann NY Acad Sci 1032: 167–178.
Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U et al (2006). Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry 59: 1208–1218.
Cohen H, Zohar J, Matar M (2003). The relevance of differential response to trauma in an animal model of post-traumatic stress disorder. Biol Psychiatry 15: 463–473.
Cohen H, Zohar J, Matar M, Loewenthal U, Kaplan Z (2007). The impact of environment factors in determining post-exposure responses in isogenic strains of mice: can genetic predisposition explain phenotypic vulnerability? Int J Neuropsychopharmacol 11: 331–349.
Cohen H, Zohar J, Matar MA, Kaplan Z, Geva AB (2005). Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biol Psychiatry 58: 640–650.
Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G (2004). Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology 29: 1962–1970.
Colmers WF, Bleakman D (1994). Effects of neuropeptide Y on the electrical properties of neurons. Trends Neurosci 17: 373–379.
Croce N, Dinallo V, Ricci V, Federici G, Caltagirone C, Bernardini S et al (2011). Neuroprotective effect of neuropeptide Y against beta-amyloid 25–35 toxicity in SH-SY5Y neuroblastoma cells is associated with increased neurotrophin production. Neurodegener Dis 8: 300–309.
de Quidt ME, Emson PC (1986). Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system-II. Immunohistochemical analysis. Neuroscience 18: 545–618.
Decressac M, Wright B, David B, Tyers P, Jaber M, Barker RA et al (2010). Exogenous neuropeptide Y promotes in vivo hippocampal neurogenesis. Hippocampus (e-pub ahead of print, 21 January 2010).
Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H et al (2010). Neuropeptide Y (NPY) gene: impact on emotional processing and treatment response in anxious depression. Eur Neuropsychopharmacol 20: 301–309.
Ehlers CL, Li TK, Lumeng L, Hwang BH, Somes C, Jimenez P et al (1998). Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res 22: 1778–1782.
File SE, Zangrossi Jr H, Sanders FL, Mabbutt PS (1993). Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol Behav 54: 1109–1111.
Flood JF, Hernandez EN, Morley JE. (1987). Modulation of memory processing by neuropeptide Y. Brain Res 421: 280–290.
Gehlert DR. (1999). Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides 33: 329–338.
Gelfo F, De Bartolo P, Tirassa P, Croce N, Caltagirone C, Petrosini L et al (2011). Intraperitoneal injection of neuropeptide Y (NPY) alters neurotrophin rat hypothalamic levels: implications for NPY potential role in stress-related disorders. Peptides 32: 1320–1323.
Goyal SN, Upadhya MA, Kokare DM, Bhisikar SM, Subhedar NK (2009). Neuropeptide Y modulates the antidepressant activity of imipramine in olfactory bulbectomized rats: involvement of NPY Y1 receptors. Brain Res 1266: 45–53.
Heilig M (2004). The NPY system in stress, anxiety and depression. Neuropeptides 38: 213–224.
Heilig M, Vecsei L, Widerlov E (1989). Opposite effects of centrally administered neuropeptide Y (NPY) on locomotor activity of spontaneously hypertensive (SH) and normal rats. Acta Physiol Scand 137: 243–248.
Heilig M, Widerlöv E (1995). Neurobiology and clinical aspects of neuropeptide Y. Crit Rev Neurobiol 9: 115–136.
Heilig M, Zachrisson O, Thorsell A, Ehnvall A, Mottagui-Tabar S, Sjogren M et al (2004). Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J Psychiatr Res 38: 113–121.
Howell OW, Silva S, Scharfman HE, Sosunov AA, Zaben M, Shatya A et al (2007). Neuropeptide Y is important for basal and seizure-induced precursor cell proliferation in the hippocampus. Neurobiol Dis 26: 174–188.
Huang EJ, Reichardt LF (2001). Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736.
Husum H, Aznar S, Hoyer-Hansen S, Larsen MH, Mikkelsen JD, Moller A et al (2006). Exacerbated loss of cell survival, neuropeptide Y-immunoreactive (IR) cells, and serotonin-IR fiber lengths in the dorsal hippocampus of the aged flinders sensitive line ‘depressed’ rat: Implications for the pathophysiology of depression? J Neurosci Res 84: 1292–1302.
Husum H, Mathé AA (2002). Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology 27: 756–764.
Jimenez-Vasquez PA, Diaz-Cabiale Z, Caberlotto L, Bellido I, Overstreet D, Fuxe K et al (2007). Electroconvulsive stimuli selectively affect behavior and neuropeptide Y (NPY) and NPY Y(1) receptor gene expressions in hippocampus and hypothalamus of Flinders Sensitive Line rat model of depression. Eur Neuropsychopharmacol 17: 298–308.
Jimenez-Vasquez PA, Mathé AA, Thomas JD, Riley EP, Ehlers CL (2001). Early maternal separation alters neuropeptide Y concentrations in selected brain regions in adult rats. Brain Res Dev Brain Res 131: 149–152.
Jones KR, Farinas I, Backus C, Reichardt LF (1994). Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 76: 989–999.
Kalra SP, Dube MG, Pu S, Xu B, Horvath TL (1999). Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20: 68–100.
Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A et al (2008). The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berl) 195: 547–557.
Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R (2002). The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev 26: 259–283.
Kask A, Rago L, Harro J (1996). Anxiogenic-like effect of the neuropeptide Y Y1 receptor antagonist BIBP 3226: antagonism with diazepam. Eur J Pharmacol 317: R3–R4.
Kim JJ, Fanselow MS, DeCola JP, Landeira-Fernandez J (1992). Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behav Neurosci 106: 591–596.
Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A et al (2002). Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience 111: 443–532.
Krysiak R, Obuchowicz E, Herman ZS (1999). Interactions between the neuropeptide Y system and the hypothalamic-pituitary-adrenal axis. Eur J Endocrinol 140: 130–136.
Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE (2000). BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci 20: 771–782.
Mathé A (1999). Neuropeptides and electroconvulsive treatment. J ECT 15: 60–75.
Mathé A, Gruber S, Jiménesz P, Theodorsson E, Stenfors C (1997). Effect of electroconvulsive stimuli and MK-801 on neuropeptide Y, neurokinin A, calcitonin gene-related peptide in rat brain. Neurochem Res 22: 629–636.
Mathé A, Husum H, El Khoury A, Jiménez-Vasquez P, Gruber S, Wörtwein G et al (2007). Search for biological correlates of depression and mechanisms of action of antidepressant treatment modalities. Do neuropeptides play a role? Physiol Behav 92: 226–231.
Mathé A, Rudorfer M, Stenfors C, Manji H, Potter W, Theodorsson E (1996). Effects of electroconvulsive treatment on somatostatin, neuropeptide Y, endothelin and neurokinin A concentration in cerebrospinal fluid of depressed patients. Depression 3: 250–257.
Mazor A, Matar MA, Kaplan Z, Kozlovsky N, Zohar J, Cohen H (2007). Gender-related qualitative differences in baseline and post-stress anxiety responses are not reflected in the incidence of criterion-based PTSD-like behaviour patterns. World J Biol Psychiatry 10: 856–869.
Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D et al (1998). International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev 50: 143–150.
Naert G, Ixart G, Maurice T, Tapia-Arancibia L, Givalois L (2011). Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol Cell Neurosci 46: 55–66.
Nawa H, Bessho Y, Carnahan J, Nakanishi S, Mizuno K (1993). Regulation of neuropeptide expression in cultured cerebral cortical neurons by brain-derived neurotrophic factor. J Neurochem 60: 772–775.
Nawa H, Pelleymounter MA, Carnahan J (1994). Intraventricular administration of BDNF increases neuropeptide expression in newborn rat brain. J Neurosci 14: 3751–3765.
Neumann ID, Wegener G, Homberg JR, Cohen H, Slattery DA, Zohar J et al (2011). Animal models of depression and anxiety: what do they tell us about human condition? Prog Neuropsychopharmacol Biol Psychiatry 35: 1357–1375.
Nikisch G, Agren H, Eap CB, Czernik A, Baumann P, Mathe AA (2005). Neuropeptide Y and corticotropin-releasing hormone in CSF mark response to antidepressive treatment with citalopram. Int J Neuropsychopharmacol 8: 403–410.
Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW (1998). Life without neuropeptide Y. Recent Prog Horm Res 53: 163–199.
Paxinos G, Watson C (2005). The Rat Brain in Stereotaxic Coordinates, 5th edn. Elsevier Academic Press: Burlington, MA.
Pickens CL, Adams-Deutsch T, Nair SG, Navarre BM, Heilig M, Shaham Y (2009). Effect of pharmacological manipulations of neuropeptide Y and corticotropin-releasing factor neurotransmission on incubation of conditioned fear. Neuroscience 164: 1398–1406.
Poo MM (2001). Neurotrophins as synaptic modulators. Nat Rev Neurosci 2: 24–32.
Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA (2005). Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology 30: 1589–1597.
Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM (2000). Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry 47: 526–539.
Redrobe JP, Dumont Y, St-Pierre JA, Quirion R (1999). Multiple receptors for neuropeptide Y in the hippocampus: putative roles in seizures and cognition. Brain Res 848: 153–166.
Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS et al (2009). Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry 66: 705–707.
Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M et al (2008). Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci 28: 893–903.
Sajdyk TJ, Vandergriff MG, Gehlert DR (1999). Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol 368: 143–147.
Schmidt MV, Liebl C, Sterlemann V, Ganea K, Hartmann J, Harbich D et al (2008). Neuropeptide Y mediates the initial hypothalamic-pituitary-adrenal response to maternal separation in the neonatal mouse. J Endocrinol 197: 421–427.
Sergeyev V, Fetissov S, Mathe AA, Jimenez PA, Bartfai T, Mortas P et al (2005). Neuropeptide expression in rats exposed to chronic mild stresses. Psychopharmacology (Berl) 178: 115–124.
Shieh PB, Ghosh A (1999). Molecular mechanisms underlying activity-dependent regulation of BDNF expression. J Neurobiol 41: 127–134.
Small CJ, Morgan DG, Meeran K, Heath MM, Gunn I, Edwards CM et al (1997). Peptide analogue studies of the hypothalamic neuropeptide Y receptor mediating pituitary adrenocorticotrophic hormone release. Proc Natl Acad Sci USA 94: 11686–11691.
Stenfors C, Theodorsson E, Mathe AA (1989). Effect of repeated electroconvulsive treatment on regional concentrations of tachykinins, neurotensin, vasoactive intestinal polypeptide, neuropeptide Y, and galanin in rat brain. J Neurosci Res 24: 445–450.
Thoenen H (2000). Neurotrophins and activity-dependent plasticity. Prog Brain Res 128: 183–191.
Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R et al (2000). Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci USA 97: 12852–12857.
Wahlestedt C, PiIch E, Koob G, Yee F, Heilig M (1993). Modulation of anxiety and neuropeptide Y-Y1 receptors by antisense oligodeoxynucleotides. Science 259: 528–531.
White J (1993). Neuropeptide Y: a central regulator of energy homeostasis. Regul Pept 49: 93–107.
Xapelli S, Bernardino L, Ferreira R, Grade S, Silva AP, Salgado JR et al (2008). Interaction between neuropeptide Y (NPY) and brain-derived neurotrophic factor in NPY-mediated neuroprotection against excitotoxicity: a role for microglia. Eur J Neurosci 27: 2089–2102.
Yehuda R, Brand S, Yang RK (2006). Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry 59: 660–663.
Acknowledgements
This study was supported by a grant from the National Institute for Psychobiology in Israel, funded by Charles E Smith Family, the Israel Academy of Science and Humanities (grant #429/03) and by the Ministry of Health (grant #6086) given to HC and the Swedish Medical Research Council grant 10414 and the Karolinska Institutet (AAM).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cohen, H., Liu, T., Kozlovsky, N. et al. The Neuropeptide Y (NPY)-ergic System is Associated with Behavioral Resilience to Stress Exposure in an Animal Model of Post-Traumatic Stress Disorder. Neuropsychopharmacol 37, 350–363 (2012). https://doi.org/10.1038/npp.2011.230
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.230
Keywords
This article is cited by
-
NAPE-PLD deletion in stress-TRAPed neurons results in an anxiogenic phenotype
Translational Psychiatry (2023)
-
Lateral hypothalamic proenkephalin neurons drive threat-induced overeating associated with a negative emotional state
Nature Communications (2023)
-
Association of Increased Amygdala Activity with Stress-Induced Anxiety but not Social Avoidance Behavior in Mice
Neuroscience Bulletin (2022)
-
Functional deletion of neuropeptide Y receptors type 2 in local synaptic networks of anteroventral BNST facilitates recall and increases return of fear
Molecular Psychiatry (2021)
-
Comparison of the Adaptive Capacity in Old and Young Wistar Rats to Stress Exposure and Acute Hypoxic Hypoxia
Bulletin of Experimental Biology and Medicine (2021)