Abstract
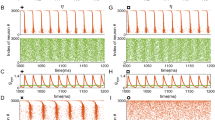
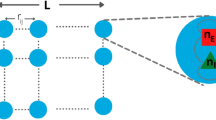
A recurrent idea in the study of complex systems is that optimal information processing is to be found near phase transitions. However, this heuristic hypothesis has few (if any) concrete realizations where a standard and biologically relevant quantity is optimized at criticality. Here we give a clear example of such a phenomenon: a network of excitable elements has its sensitivity and dynamic range maximized at the critical point of a non-equilibrium phase transition. Our results are compatible with the essential role of gap junctions in olfactory glomeruli and retinal ganglionar cell output. Synchronization and global oscillations also emerge from the network dynamics. We propose that the main functional role of electrical coupling is to provide an enhancement of dynamic range, therefore allowing the coding of information spanning several orders of magnitude. The mechanism could provide a microscopic neural basis for psychophysical laws.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Stevens, S. S. Psychophysics: Introduction to its Perceptual, Neural and Social Prospects (Wiley, New York, 1975).
Wachowiak, M. & Cohen, L. B. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 32, 723–735 (2001).
Angioy, A. M., Desogus, A., Barbarossa, I. T., Anderson, P. & Hansson, B. S. Extreme sensitivity in an olfactory system. Chem. Senses 28, 279–284 (2003).
Fried, H. U., Fuss, S. H. & Korsching, S. I. Selective imaging of presynaptic activity in the mouse olfactory bulb shows concentration and structure dependence of odor responses in identified glomeruli. Proc. Natl Acad. Sci. USA 99, 3222–3227 (2002).
Cleland, T. A. & Linster, C. Concentration tuning mediated by spare receptor capacity in olfactory sensory neurons: a theoretical study. Neural Comput. 11, 1673–1690 (1999).
Copelli, M., Roque, A. C., Oliveira, R. F. & Kinouchi, O. Physics of psychophysics: Stevens and Weber-Fechner laws are transfer functions of excitable media. Phys. Rev. E 65, 060901 (2002).
Copelli, M. & Kinouchi, O. Intensity coding in two-dimensional excitable neural networks. Physica A 349, 431–442 (2005).
Copelli, M., Oliveira, R. F., Roque, A. C. & Kinouchi, O. Signal compression in the sensory periphery. Neurocomputing 65–66, 691–696 (2005).
Reiser, J. & Matthews, H. Response properties of isolated mouse olfactory receptor cells. J. Physiol. 530, 113–122 (2001).
Tomaru, A. & Kurahashi, T. Mechanisms determining the dynamic range of the bullfrog olfactory receptor cell. J. Neurophysiol. 93, 1880–1888 (2005).
Chater, N. & Brown, G. D. Scale-invariance as a unifying psychological principle. Cognition 69, B17–B24 (1999).
Furtado, L. S. & Copelli, M. Response of electrically coupled spiking neurons: a cellular automaton approach. Phys. Rev. E 73, 011907 (2006).
Beggs, J. M. & Plenz, D. Neuronal avalanches in neocortical circuits. J. Neurosci. 23, 11167–11177 (2003).
Haldeman, C. & Beggs, J. M. Critical branching captures activity in living neural networks and maximizes the number of metastable states. Phys. Rev. Lett. 94, 058101 (2005).
Langton, C. G. Computation at the edge of chaos: phase transitions and emergent computation. Physica D 42, 12–37 (1990).
Bak, P. How Nature Works: The Science of Self-Organized Criticality (Oxford Univ. Press, New York, 1997).
Chialvo, D. R. Critical brain networks. Physica A 340, 756–765 (2004).
Kosaka, T., Deans, M. R., Paul, D. L. & Kosaka, K. Neuronal gap junctions in the mouse main olfactory bulb: morphological analyses on transgenic mice. Neuroscience 134, 757–769 (2005).
Migliore, M., Hines, M. L. & Shepherd, G. M. The role of distal dendritic gap junctions in synchronization of mitral cell axonal output. J. Comput. Neurosci. 18, 151–161 (2005).
Christie, J. M. et al. Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron 46, 761–772 (2005).
Marro, J. & Dickman, R. Nonequilibrium Phase Transitions in Lattice Models (Cambridge Univ. Press, Cambridge, 1999).
Laurent, G. Olfactory network dynamics and the coding of multidimensional signals. Nature Rev. Neurosci. 3, 884–895 (2002).
Lewis, T. J. & Rinzel, J. Topological target patterns and population oscillations in a network with random gap junctional coupling. Neurocomputing 38–40, 763–768 (2001).
Lewis, T. J. & Rinzel, J. Self-organized synchronous oscillations in a network of excitable cells coupled by gap junctions. Network Comput. Neural Syst. 11, 299–320 (2000).
Schubert, T. et al. Connexin36 mediates gap junctional coupling of alpha-ganglion cells in mouse retina. J. Comp. Neurol. 485, 191–201 (2005).
Hidaka, S., Akahori, Y. & Kurosawa, Y. Cellular/molecular dendrodendritic electrical synapses between mammalian retinal ganglion cells. J. Neurosci. 24, 10553–10567 (2005).
Vogt, A., Hormuzdi, S. G. & Monyer, H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res. Mol. Brain Res. 141, 113–120 (2005).
Deans, M. R., Volgyi, B., Goodenough, D. A., Bloomfield, S. A. & Paul, D. L. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron 36, 703–712 (2002).
Zhang, C. & Restrepo, D. Expression of connexin 45 in the olfactory system. Brain Res. 929, 37–47 (2002).
Camalet, S., Duke, T., Jülicher, F. & Prost, J. Auditory sensitivity provided by self-tuned critical oscillations of hair cells. Proc. Natl Acad. Sci. USA 97, 3183–3188 (2000).
Sohl, G., Maxeiner, S. & Willecke, K. Expression and functions of neuronal gap junctions. Nature Rev. Neurosci. 6, 191–200 (2005).
Acknowledgements
This research is supported by CNPq, FACEPE, CAPES and PRONEX. The authors are grateful for discussions with A. C. Roque, R. F. Oliveira, D. Restrepo, T. Cleland and V. R. Vitorino de Assis and for encouragement from N. Caticha.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kinouchi, O., Copelli, M. Optimal dynamical range of excitable networks at criticality. Nature Phys 2, 348–351 (2006). https://doi.org/10.1038/nphys289
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys289
This article is cited by
-
Neuronal connected burst cascades bridge macroscale adaptive signatures across arousal states
Nature Communications (2023)
-
Brain-wide dendrites in a near-optimal performance of dynamic range and information transmission
Scientific Reports (2023)
-
Fish shoals resemble a stochastic excitable system driven by environmental perturbations
Nature Physics (2023)
-
Collective excitations of germinating pollen grains at critical points
Scientific Reports (2023)
-
Critical dynamics arise during structured information presentation within embodied in vitro neuronal networks
Nature Communications (2023)