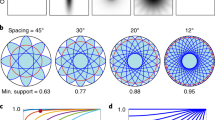
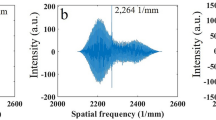
Abstract
High-resolution real-time tomography of scattering tissues is important for many areas of medicine and biology1,2,3,4,5,6. However, the compromise between transverse resolution and depth-of-field, in addition to low sensitivity deep in tissue, continues to impede progress towards cellular-level volumetric tomography. Computed imaging has the potential to solve these long-standing limitations. Interferometric synthetic aperture microscopy7,8,9 is a computed imaging technique enabling high-resolution volumetric tomography with spatially invariant resolution. However, its potential for clinical diagnostics remains largely untapped because full volume reconstructions required lengthy post-processing, and the phase-stability requirements have been difficult to satisfy in vivo. Here, we demonstrate how three-dimensional Fourier-domain resampling, in combination with high-speed optical coherence tomography, can achieve high-resolution in vivo tomography. Enhanced depth sensitivity was achieved over a depth of field extended in real time by more than an order of magnitude. This work lays the foundation for high-speed volumetric cellular-level tomography.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Boppart, S. A. et al. In vivo cellular optical coherence tomography imaging. Nature Med. 4, 861–865 (1998).
Adler, D. C. et al. Three-dimensional endomicroscopy using optical coherence tomography. Nature Photon. 1, 709–716 (2007).
Liu, L. et al. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nature Med. 17, 1010–1014 (2011).
Vakoc, B. J. et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nature Med. 15, 1219–1223 (2009).
Yun, S. H. et al. Comprehensive volumetric optical microscopy in vivo. Nature Med. 12, 1429–1433 (2006).
Drexler, W. et al. Ultrahigh-resolution ophthalmic optical coherence tomography. Nature Med. 7, 502–507 (2001).
Ralston, T. S., Marks, D. L., Carney, P. S. & Boppart, S. A. Interferometric synthetic aperture microscopy. Nature Phys. 3, 129–134 (2007).
Davis, B. J., Marks, D. L., Ralston, T. S., Carney, P. S. & Boppart, S. A. Interferometric synthetic aperture microscopy: computed imaging for scanned coherent microscopy. Sensors 8, 3903–3931 (2008).
Davis, B. J. et al. Nonparaxial vector-field modeling of optical coherence tomography and interferometric synthetic aperture microscopy. J. Opt. Soc. Am. A 24, 2527–2542 (2007).
Srinivasan, V. J., Radhakrishnan, H., Jiang, J. Y., Barry, S. & Cable, A. E. Optical coherence microscopy for deep tissue imaging of the cerebral cortex with intrinsic contrast. Opt. Express 20, 2220–2239 (2012).
Rolland, J. P., Meemon, P., Murali, S., Thompson, K. P. & Lee, K-S. Gabor-based fusion technique for optical coherence microscopy. Opt. Express 18, 3632–3642 (2010).
Rajadhyaksha, M., Gonzalez, S., Zavislan, J. M., Anderson, R. R. & Webb, R. H. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J. Invest. Dermatol. 113, 293–303 (1999).
Beau, A. S. et al. In vivo endoscopic multi-beam optical coherence tomography. Phys. Med. Biol. 55, 615–622 (2010).
Leitgeb, R. A., Villiger, M., Bachmann, A. H., Steinmann, L. & Lasser, T. Extended focus depth for Fourier domain optical coherence microscopy. Opt. Lett. 31, 2450–2452 (2006).
Blatter, C. et al. Extended focus high-speed swept source OCT with self-reconstructive illumination. Opt. Express 19, 12141–12155 (2011).
Yasuno, Y. et al. Non-iterative numerical method for laterally superresolving Fourier domain optical coherence tomography. Opt. Express 14, 1006–1020 (2006).
Yu, L. et al. Improved lateral resolution in optical coherence tomography by digital focusing using two-dimensional numerical diffraction method. Opt. Express 15, 7634–7641 (2007).
Colomb, T. et al. Numerical parametric lens for shifting, magnification, and complete aberration compensation in digital holographic microscopy. J. Opt. Soc. Am. A 23, 3177–3190 (2006).
Adie, S. G., Graf, B. W., Ahmad, A., Carney, P. S. & Boppart, S. A. Computational adaptive optics for broadband optical interferometric tomography of biological tissue. Proc. Natl Acad. Sci. USA 109, 7175–7180 (2012).
Hillmann, D., Franke, G., Lührs, C., Koch, P. & Hüttmann, G. Efficient holoscopy image reconstruction. Opt. Express 20, 21247–21263 (2012).
Kim, M-K. Tomographic three-dimensional imaging of a biological specimen using wavelength-scanning digital interference holography. Opt. Express 7, 305–310 (2000).
Ralston, T. S., Marks, D. L., Carney, P. S. & Boppart, S. A. Real-time interferometric synthetic aperture microscopy. Opt. Express 16, 2555–2569 (2008).
Wilke, K., Martin, A., Terstegen, L. & Biel, S. S. A short history of sweat gland biology. Int. J. Cosmetic Sci. 29, 169–179 (2007).
Gabarda, S. & Cristóbal, G. Blind image quality assessment through anisotropy. J. Opt. Soc. Am. A 24, B42–B51 (2007).
Blatter, C. et al. In situ structural and microangiographic assessment of human skin lesions with high-speed OCT. Biomed. Opt. Express 3, 2636–2646 (2012).
Acknowledgements
The authors thank E.J. Chaney (University of Illinois at Urbana-Champaign) for providing the tissue samples used throughout this study, D.L. Marks (formerly at University of Illinois at Urbana-Champaign) for providing the tissue phantom and D. Spillman (University of Illinois at Urbana-Champaign) for administrative and information technology support related to this research. This research was supported in part by grants from the National Institutes of Health (NIH; R01 EB012479 to S.A.B.) and an NIH Bioengineering Research Partnership (R01 EB013723 to S.A.B.). A.A. was funded in part by the NIH National Cancer Institute Alliance for Nanotechnology in Cancer (Midwest Cancer Nanotechnology Training Center; grant R25-CA154015A) and Texas Instruments.
Author information
Authors and Affiliations
Contributions
Experimental data were acquired by A.A., N.D.S. and S.G.A. Analysis and interpretation of data was carried out by A.A., N.D.S. and S.G.A. Additional processing and visualization was performed by A.A. and N.D.S. GPU code was written by A.A., N.D.S. and H.K., and reviewed by W.W.H. The manuscript was written and edited by all authors. S.A.B. and P.S.C. made seminal contributions to the core ideas carried out in this study, and obtained funding to support this research.
Corresponding author
Ethics declarations
Competing interests
S.A.B. and P.S.C are co-founders of Diagnostic Photonics, Inc., which is licensing intellectual property from the University of Illinois at Urbana-Champaign related to Interferometric Synthetic Aperture Microscopy. S.A.B. received patent royalties from the Massachusetts Institute of Technology for technology related to optical coherence tomography. Other authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1180 kb)
Supplementary video
Supplementary video (MOV 9588 kb)
Supplementary video
Supplementary video (MOV 5086 kb)
Rights and permissions
About this article
Cite this article
Ahmad, A., Shemonski, N., Adie, S. et al. Real-time in vivo computed optical interferometric tomography. Nature Photon 7, 444–448 (2013). https://doi.org/10.1038/nphoton.2013.71
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2013.71
This article is cited by
-
Label-free metabolic and structural profiling of dynamic biological samples using multimodal optical microscopy with sensorless adaptive optics
Scientific Reports (2022)
-
Resolution-enhanced OCT and expanded framework of information capacity and resolution in coherent imaging
Scientific Reports (2021)
-
Cellular-resolution in vivo tomography in turbid tissue through digital aberration correction
PhotoniX (2020)
-
Angular compounding for speckle reduction in optical coherence tomography using geometric image registration algorithm and digital focusing
Scientific Reports (2020)
-
Disintegration of simulated drinking water biofilms with arrays of microchannel plasma jets
npj Biofilms and Microbiomes (2018)