Abstract
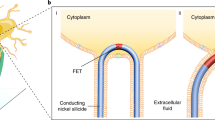
The ability to make electrical measurements inside cells has led to many important advances in electrophysiology1,2,3,4,5,6. The patch clamp technique, in which a glass micropipette filled with electrolyte is inserted into a cell, offers both high signal-to-noise ratio and temporal resolution1,2. Ideally, the micropipette should be as small as possible to increase the spatial resolution and reduce the invasiveness of the measurement, but the overall performance of the technique depends on the impedance of the interface between the micropipette and the cell interior1,2, which limits how small the micropipette can be. Techniques that involve inserting metal or carbon microelectrodes into cells are subject to similar constraints4,7,8,9. Field-effect transistors (FETs) can also record electric potentials inside cells10, and because their performance does not depend on impedance11,12, they can be made much smaller than micropipettes and microelectrodes. Moreover, FET arrays are better suited for multiplexed measurements. Previously, we have demonstrated FET-based intracellular recording with kinked nanowire structures10, but the kink configuration and device design places limits on the probe size and the potential for multiplexing. Here, we report a new approach in which a SiO2 nanotube is synthetically integrated on top of a nanoscale FET. This nanotube penetrates the cell membrane, bringing the cell cytosol into contact with the FET, which is then able to record the intracellular transmembrane potential. Simulations show that the bandwidth of this branched intracellular nanotube FET (BIT-FET) is high enough for it to record fast action potentials even when the nanotube diameter is decreased to 3 nm, a length scale well below that accessible with other methods1,2,4. Studies of cardiomyocyte cells demonstrate that when phospholipid-modified BIT-FETs are brought close to cells, the nanotubes can spontaneously penetrate the cell membrane to allow the full-amplitude intracellular action potential to be recorded, thus showing that a stable and tight seal forms between the nanotube and cell membrane. We also show that multiple BIT-FETs can record multiplexed intracellular signals from both single cells and networks of cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sakmann, B. & Neher, E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu. Rev. Physiol. 46, 455–472 (1984).
Molleman, A. Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology (Wiley, 2003).
Rutten, W. L. C. Selective electrical interfaces with the nervous system. Annu. Rev. Biomed. Engl. 4, 407–452 (2002).
Purves, R. D. Microelectrode Methods for Intracellular Recording and Ionophoresis (Academic Press, 1981).
Chorev, E., Epsztein, J., Houweling, A. R., Lee, A. K. & Brecht, M. Electrophysiological recordings from behaving animals—going beyond spikes. Curr. Opin. Neurobiol. 19, 513–519 (2009).
Dunlop, J., Bowlby, M., Peri, R., Vasilyev, D. & Arias, R. High-throughput electrophysiology: an emerging paradigm for ion-channel screening and physiology. Nature Rev. Drug Discov. 7, 358–368 (2008).
Hai, A., Shappir, J. & Spira, M. E. In-cell recordings by extracellular microelectrodes. Nature Methods 7, 200–202 (2010).
Schrlau, M. G., Dun, N. J. & Bau, H. H. Cell electrophysiology with carbon nanopipettes. ACS Nano 3, 563–568 (2009).
De Asis, E. D., Leung, J., Wood, S. & Nguyen, C. V. High spatial resolution single multiwalled carbon nanotube electrode for stimulation, recording, and whole cell voltage clamping of electrically active cells. Appl. Phys. Lett. 95, 153701 (2009).
Tian, B. et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329, 831–834 (2010).
Sze, S. M. & Ng, K. K. Physics of Semiconductor Devices 3rd edn (Wiley Interscience, 2006).
Patolsky, F., Zheng, G. & Lieber, C. M. Nanowire-based biosensors. Anal. Chem. 78, 4260–4269 (2006).
Jiang, X. et al. Rational growth of branched nanowire heterostructures with synthetically-encoded properties and function. Proc. Natl Acad. Sci. USA 108, 12212–12216 (2011).
Hausmann, D., Becker, J., Wang, S. & Gordon, R. G. Rapid vapor deposition of highly conformal silica nanolaminates. Science 298, 402–406 (2002).
Sadiku, M. N. O. Elements of Electromagnetics 3rd edn (Oxford Univ. Press, 2000).
Cohen-Karni, T., Timko, B. P., Weiss, L. E. & Lieber, C. M. Flexible electrical recording from cells using nanowire transistor arrays. Proc. Natl Acad. Sci. USA 106, 7309–7313 (2009).
Scanziani, M. & Hausser, M. Electrophysiology in the age of light. Nature 461, 930–939 (2009).
Davie, J. T. et al. Dendritic patch-clamp recording. Nature Protoc. 1, 1235–1247 (2006).
Bers, D. M. Cardiac excitation–contraction coupling. Nature 415, 198–205 (2002).
Zipes, D. P. & Jalife, J. Cardiac Electrophysiology: From Cell to Bedside 5th edn (Saunders, 2009).
Buck, R. P. & Grabbe, E. S. Electrostatic and thermodynamic analysis of suspension effect potentiometry. Anal. Chem. 58, 1938–1941 (1986).
Tasaki, I. & Singer, I. Some problems involved in electric measurements of biological systems. Ann. NY Acad. Sci. 148, 36–53 (1968).
Chernomordik, L. V. & Kozlov, M. M. Mechanics of membrane fusion. Nature Struct. Mol. Biol. 15, 675–683 (2008).
Almquist, B. D. & Melosh, N. A. Fusion of biomimetic stealth probes into lipid bilayer cores. Proc. Natl Acad. Sci. USA 107, 5815–5820 (2010).
Yan, H. et al. Programmable nanowire circuits for nanoprocessors. Nature 470, 240–244 (2011).
International Technology Roadmap for Semiconductors (2009); available online at http://www.itrs.net.
Acknowledgements
The authors thank Z. Jiang and H. Yan for helpful discussions. R.G. acknowledges a Japan Student Services Organization Graduate Research Fellowship. C.M.L. acknowledges a NIH Director's Pioneer Award (5DP1OD003900).
Author information
Authors and Affiliations
Contributions
X.D. and C.M.L. designed the experiments. X.D., R.G., T.C-K., Q.Q., H.S.C. and B.T. performed experiments. X.D., P.X. and Q.Q. performed modelling and analyses. X.D., P.X., Q.Q., X.J. and C.M.L. analysed data. X.D., P.X. and C.M.L. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1099 kb)
Rights and permissions
About this article
Cite this article
Duan, X., Gao, R., Xie, P. et al. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nature Nanotech 7, 174–179 (2012). https://doi.org/10.1038/nnano.2011.223
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2011.223
This article is cited by
-
Electrochemical field-effect bio-transistor based on a multi-scale electronic nanomesh of single-walled carbon nanotubes
Carbon Letters (2023)
-
Quantification of Cardiomyocyte Contraction In Vitro and Drug Screening by MyocytoBeats
Journal of Cardiovascular Translational Research (2023)
-
Nanocrown electrodes for parallel and robust intracellular recording of cardiomyocytes
Nature Communications (2022)
-
Biointerface design for vertical nanoprobes
Nature Reviews Materials (2022)
-
Semi-Implantable Bioelectronics
Nano-Micro Letters (2022)