Abstract
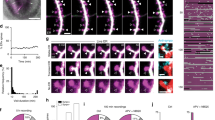
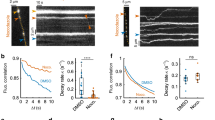
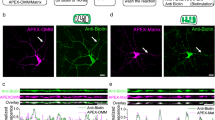
The regulated trafficking of neurotransmitter receptors at synapses is critical for synaptic function and plasticity. However, the molecular machinery that controls active transport of receptors into synapses is largely unknown. We found that, in rat hippocampus, the insertion of AMPA receptors (AMPARs) into spines during synaptic plasticity requires a specific motor protein, which we identified as myosin Va. We found that myosin Va associates with AMPARs through its cargo binding domain. This interaction was enhanced by active, GTP-bound Rab11, which is also transported by the motor protein. Myosin Va mediated the CaMKII-triggered translocation of GluR1 receptors from the dendritic shaft into spines, but it was not required for constitutive GluR2 trafficking. Accordingly, myosin Va was specifically required for long-term potentiation, but not for basal synaptic transmission. In summary, we identified the specific motor protein and organelle acceptor that catalyze the directional transport of AMPARs into spines during activity-dependent synaptic plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kennedy, M.J. & Ehlers, M.D. Organelles and trafficking machinery for postsynaptic plasticity. Annu. Rev. Neurosci. 29, 325–362 (2006).
Park, M. et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 52, 817–830 (2006).
Shi, S.H. et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284, 1811–1816 (1999).
Kim, J., Jung, S.C., Clemens, A.M., Petralia, R.S. & Hoffman, D.A. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron 54, 933–947 (2007).
Shepherd, J.D. & Huganir, R.L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 23, 613–643 (2007).
Passafaro, M., Piech, V. & Sheng, M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci. 4, 917–926 (2001).
Shi, S., Hayashi, Y., Esteban, J.A. & Malinow, R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343 (2001).
Luscher, C. et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron 24, 649–658 (1999).
Nishimune, A. et al. NSF binding to GluR2 regulates synaptic transmission. Neuron 21, 87–97 (1998).
Song, I. et al. Interaction of the N-ethylmaleimide–sensitive factor with AMPA receptors. Neuron 21, 393–400 (1998).
Hayashi, Y. et al. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 (2000).
Carlisle, H.J. & Kennedy, M.B. Spine architecture and synaptic plasticity. Trends Neurosci. 28, 182–187 (2005).
Karcher, R.L., Deacon, S.W. & Gelfand, V.I. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 12, 21–27 (2002).
Osterweil, E., Wells, D.G. & Mooseker, M.S. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J. Cell Biol. 168, 329–338 (2005).
Lise, M.F. et al. Involvement of myosin Vb in glutamate receptor trafficking. J. Biol. Chem. 281, 3669–3678 (2006).
Triller, A. & Choquet, D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move!. Trends Neurosci. 28, 133–139 (2005).
Mercer, J.A., Seperack, P.K., Strobel, M.C., Copeland, N.G. & Jenkins, N.A. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature 349, 709–713 (1991).
Naisbitt, S. et al. Interaction of the postsynaptic density–95/guanylate kinase domain–associated protein complex with a light chain of myosin-V and dynein. J. Neurosci. 20, 4524–4534 (2000).
Walikonis, R.S. et al. Identification of proteins in the postsynaptic density fraction by mass spectrometry. J. Neurosci. 20, 4069–4080 (2000).
Yoshimura, A. et al. myosin-Va facilitates the accumulation of mRNA/protein complex in dendritic spines. Curr. Biol. 16, 2345–2351 (2006).
Pastural, E. et al. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat. Genet. 16, 289–292 (1997).
Sanal, O. et al. An allelic variant of Griscelli disease: presentation with severe hypotonia, mental-motor retardation and hypopigmentation consistent with Elejalde syndrome (neuroectodermal melanolysosomal disorder). J. Neurol. 247, 570–572 (2000).
Tabb, J.S., Molyneaux, B.J., Cohen, D.L., Kuznetsov, S.A. & Langford, G.M. Transport of ER vesicles on actin filaments in neurons by myosin V. J. Cell Sci. 111, 3221–3234 (1998).
Takagishi, Y. et al. The dilute lethal (dl) gene attacks a Ca2+ store in the dendritic spine of Purkinje cells in mice. Neurosci. Lett. 215, 169–172 (1996).
Petralia, R.S. et al. Glutamate receptor targeting in the postsynaptic spine involves mechanisms that are independent of myosin Va. Eur. J. Neurosci. 13, 1722–1732 (2001).
Miyata, M. et al. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron 28, 233–244 (2000).
Schnell, E. & Nicoll, R.A. Hippocampal synaptic transmission and plasticity are preserved in myosin Va mutant mice. J. Neurophysiol. 85, 1498–1501 (2001).
Wenthold, R.J., Petralia, R.S., Blahos, J., II & Niedzielski, A.S. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci. 16, 1982–1989 (1996).
Ullrich, O., Reinsch, S., Urbe, S., Zerial, M. & Parton, R.G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913–924 (1996).
Brown, J.R., Simonetta, K.R., Sandberg, L.A., Stafford, P. & Langford, G.M. Recombinant globular tail fragment of myosin-V blocks vesicle transport in squid nerve cell extracts. Biol. Bull. 201, 240–241 (2001).
Lapierre, L.A. et al. myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell 12, 1843–1857 (2001).
Rodriguez, O.C. & Cheney, R.E. Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J. Cell Sci. 115, 991–1004 (2002).
Zhu, J.J., Esteban, J.A., Hayashi, Y. & Malinow, R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat. Neurosci. 3, 1098–1106 (2000).
Watt, A.J., van Rossum, M.C., MacLeod, K.M., Nelson, S.B. & Turrigiano, G.G. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron 26, 659–670 (2000).
Gerges, N.Z., Brown, T.C., Correia, S.S. & Esteban, J.A. Analysis of Rab protein function in neurotransmitter receptor trafficking at hippocampal synapses. Methods Enzymol. 403, 153–166 (2005).
Ehrlich, I. & Malinow, R. Postsynaptic density–95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J. Neurosci. 24, 916–927 (2004).
Piccini, A. & Malinow, R. Critical postsynaptic density–95/disc large/zonula occludens–1 interactions by glutamate receptor 1 (GluR1) and GluR2 required at different subcellular sites. J. Neurosci. 22, 5387–5392 (2002).
Ryu, J. et al. A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron 49, 175–182 (2006).
Park, M., Penick, E.C., Edwards, J.G., Kauer, J.A. & Ehlers, M.D. Recycling endosomes supply AMPA receptors for LTP. Science 305, 1972–1975 (2004).
Brown, T.C., Correia, S.S., Petrok, C.N. & Esteban, J.A. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J. Neurosci. 27, 13311–13315 (2007).
Seabra, M.C. & Coudrier, E. Rab GTPases and myosin motors in organelle motility. Traffic 5, 393–399 (2004).
Jordens, I., Marsman, M., Kuijl, C. & Neefjes, J. Rab proteins, connecting transport and vesicle fusion. Traffic 6, 1070–1077 (2005).
Krementsov, D.N., Krementsova, E.B. & Trybus, K.M. myosin V: regulation by calcium, calmodulin and the tail domain. J. Cell Biol. 164, 877–886 (2004).
Lynch, G., Larson, J., Kelso, S., Barrionuevo, G. & Schottler, F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 305, 719–721 (1983).
Barral, D.C. et al. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J. Clin. Invest. 110, 247–257 (2002).
Cao, T.T., Chang, W., Masters, S.E. & Mooseker, M.S. myosin-Va binds to and mechanochemically couples microtubules to actin filaments. Mol. Biol. Cell 15, 151–161 (2004).
Ali, M.Y. et al. myosin Va maneuvers through actin intersections and diffuses along microtubules. Proc. Natl. Acad. Sci. USA 104, 4332–4336 (2007).
Huang, J.D. et al. Direct interaction of microtubule- and actin-based transport motors. Nature 397, 267–270 (1999).
Mehta, A.D. et al. myosin-V is a processive actin-based motor. Nature 400, 590–593 (1999).
Dunah, A.W. et al. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat. Neurosci. 8, 458–467 (2005).
Acknowledgements
We wish to dedicate this work to the memory of Alaa El-Husseini, a great friend and an outstanding scientist. We thank T. Robinson and J. Jedynak for the use of the Neurolucida software, V. Gelfand and R. Holz for the plasmid containing the mouse myosin Va globular tail, D. Wells and M. Mooseker for the human myosin VI, Y. Takagishi for the chicken full-length myosin Va, S. Lee for letting us test an unpublished siRNA against myosin Va (different from the one shown in this study) and R. Malinow for the PSD-95 construct. We also thank L. Weisman, M. Meisler and R. Holz for their comments on this work. This work was supported by grants from the US National Institute of Mental Health (MH070417 to J.A.E. and F31-MH070205 to T.C.B.), Fundação para a Ciência e a Tecnologia (SFRH/BPD/14620 to S.S.C.) and the European Community (LSHM-CT-2004-511995, SYNSCAFF to M.P.).
Author information
Authors and Affiliations
Contributions
S.S.C. is responsible for most of the experimental work. S.S.C. and J.A.E. designed the experiments and wrote the manuscript. S.B carried out the biochemical experiments of Figure 1 and the immunocytochemistry in Figure 2a,b. T.C.B. contributed some of the electrophysiology and imaging experiments. M.-F.L. developed and characterized the siRNA used in this study. D.S.B. carried out some of the initial cloning of this project. A.E.-H. and M.P. designed and supervised the experiments carried out by M.-F.L. and S.B., respectively.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Methods (PDF 1326 kb)
Rights and permissions
About this article
Cite this article
Correia, S., Bassani, S., Brown, T. et al. Motor protein–dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci 11, 457–466 (2008). https://doi.org/10.1038/nn2063
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2063
This article is cited by
-
Myosin Va-dependent Transport of NMDA Receptors in Hippocampal Neurons
Neuroscience Bulletin (2024)
-
Effects of DeSUMOylated Spastin on AMPA Receptor Surface Delivery and Synaptic Function Are Enhanced by Phosphorylating at Ser210
Molecular Neurobiology (2024)
-
Endosomal traffic and glutamate synapse activity are increased in VPS35 D620N mutant knock-in mouse neurons, and resistant to LRRK2 kinase inhibition
Molecular Brain (2021)
-
Endoplasmic reticulum visits highly active spines and prevents runaway potentiation of synapses
Nature Communications (2020)
-
Molecular Mechanisms of Early and Late LTP
Neurochemical Research (2019)