Abstract
At the developing neuromuscular junction, motor neuron–derived agrin triggers the differentiation of postsynaptic membrane into a highly specialized structure, where the nicotinic acetylcholine receptors (AChRs) are aggregated into high-density clusters. Agrin acts by activating the muscle-specific kinase MuSK and inducing coaggregation of the 43-kDa protein rapsyn with AChRs on muscle cell membrane1,2. The signaling mechanism downstream of MuSK is poorly defined. We report here that the mouse tumor suppressor protein adenomatous polyposis coli (APC) has a role in AChR clustering and that the Wnt/β-catenin pathway may crosstalk with agrin signaling cascade during synapse formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hall, Z.W. & Sanes, J.R. Cell 72 (Suppl.), 99–121 (1993).
Sanes, J.R. & Lichtman, J.W. Nat. Rev. Neurosci. 2, 791–805 (2001).
Wallace, B.G., Qu, Z. & Huganir, R.L. Neuron 6, 869–878 (1991).
Dai, Z., Luo, X., Xie, H. & Peng, H.B. J. Cell Biol. 150, 1321–1334 (2000).
Borges, L.S. & Ferns, M. J. Cell Biol. 153, 1–12 (2001).
Wang, J.M. et al. Nat. Neurosci. 5, 963–970 (2002).
Su, L.-K. et al. Science 256, 668–670 (1992).
Ferns, M. et al. Neuron 8, 1079–1086 (1992).
Bienz, M. Nat. Rev. Mol. Cell. Biol. 3, 328–338 (2002).
Rosin-Arbesfeld, R., Ihrke, G. & Bienz, M. EMBO J. 20, 5929–5939 (2001).
Nathke, I.S., Adams, C.L., Polakis, P., Sellin, J.H. & Nelson, W.J. J. Cell Biol. 134, 165–179 (1996).
Jasmin, B.J., Changeux, J.P. & Cartaud, J. Nature 344, 673–675 (1990).
Hall, A.C., Lucas, F.R. & Salinas, P.C. Cell 100, 525–535 (2000).
Packard, M. et al. Cell 111, 319–330 (2002).
Acknowledgements
We thank E. Aizenman, E. Frank and W. Halfter for advice. This work was supported by grants from US National Institutes of Health and Muscular Dystrophy Association to Z.Z.W.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1.
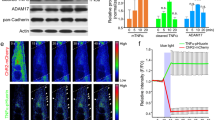
Association of AChR b subunit with APC. (a) The large cytoplasmic loop of βsubunit is located between the third (TM3) and fourth (TM4) transmembrane segments, encompassing amino-acid residues 333 to 469. The letter Y denotes potential tyrosine residues that may be phosphorylated. Numbers refer to residues at the boundaries of each construct. The C-terminal portion of the βloop (residues 406-469) mediated the interaction of β subunit with APC in the yeast two-hybrid assay. (b) A Coomassie blue-stained SDS gel showing specific binding of GST-β fusion proteins with the 6 x histidine-tagged APC clone. (c) The AChRs were pulled down using mAb124 or α-BTX-Sepharose from detergent extracts of the Tibialis anterior muscle of 4 week-old mice. Bound APC proteins were immunoblotted with the polyclonal antibody against APC. In the bottom panel, APC was first immunoprecipitated, and the precipitates were blotted using mAb124 against the βsubunit. Control experiments for the immunoprecipitation were carried out using normal mouse serum, Sepharose-4B resin, or the APC antibody preabsorbed with the immunizing peptide. (JPG 17 kb)
Supplementary Fig. 2.
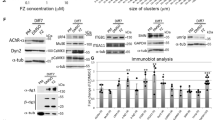
Interaction between APC and AChR is required for AChR clustering induced by agrin. (a) C2 myoblasts were stably transfected with cDNAs encoding protein fragments of APC and the β loop fused to GFP. Forty-eight hours after growth in the differentiation medium, the cells fused to form large myotubes, which expressed high levels of the fusion proteins in the cytoplasm. (b) Effects of overexpression of the GFP fusion proteins on agrin-induced binding of APC to the AChR. AChRs were pulled-down using α-BTX-Sepharose from detergent extracts of muscle cell cultures treated with neural agrin (10 nM, 4h). Co-precipitated APC and AChR proteins were immunoblotted with anti-APC and mAb124, respectively. (c) The stable cell lines were incubated with or without neural agrin (10 nM, 4h), and then stained with rhodamine-α-BTX to label AChR clusters. (d) To ascertain that the inhibitory effect of GFP-APC1998-2170 and β406-469 was not due to the loss of ability of the muscle cells to aggregate AChR upon prolonged passaging, we isolated reversants of myoblasts that have lost the constructs after growth in the absence of G418 for three passages. In the absence of the dominant inhibitory proteins, the AChR-clustering activity of myotubes resumed upon stimulation by neural agrin (10 nM, 4 h). (e) Overexpression of the GFP-APC and GFP-β fusion proteins did not change the levels of AChR expression on the cell surface as determined by 125I-a-BTX binding assay. (JPG 49 kb)
Supplementary Fig. 3.
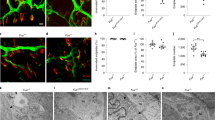
Agrin induced MuSK activation. C2C12 myotubes overexpressing the GFP constructs were incubated with or without neural agrin (CAg12,4,8, 10 nM) for 1 h at 37° C. MuSK was isolated from cell extracts by immunoprecipitation using a rabbit anti-MuSK antibody 10. Precipitated proteins were separated by SDS-PAGE, transferred onto nitrocellulose filter, and probed with the anti-phosphotyrosine antibody mAb4G10. No phosphotyrosine signal was observed in lanes containing untreated muscle extracts. In contrast, strongly labeled phosphotyrosine bands corresponding to MuSK were observed in all three lanes containing extracts treated with neural agrin. The blots were stripped and reprobed with a goat antiserum against MuSK (N-19) to show that the amount of MuSK in each lane is similar. The arrow indicates the MuSK-reactive band that corresponds to the single band in the phosphotyrosine blot above. (JPG 15 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Jing, Z., Zhang, L. et al. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat Neurosci 6, 1017–1018 (2003). https://doi.org/10.1038/nn1128
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1128
This article is cited by
-
Alteration of actin cytoskeletal organisation in fetal akinesia deformation sequence
Scientific Reports (2024)
-
WNTs in synapse formation and neuronal circuitry
The EMBO Journal (2012)
-
Colocalization of APC and PSD-95 in the nerve fiber as well as in the post-synapse of matured neurons
Medical Molecular Morphology (2012)
-
WNTs tune up the neuromuscular junction
Nature Reviews Neuroscience (2009)
-
β-catenin in reverse action
Nature Neuroscience (2008)