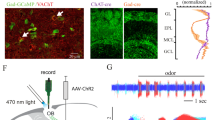
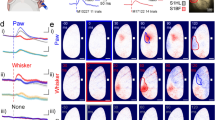
Abstract
The basal forebrain provides the primary source of cholinergic input to the cortex, and it has a crucial function in promoting wakefulness and arousal. However, whether rapid changes in basal forebrain neuron spiking in awake animals can dynamically influence sensory perception is unclear. Here we show that basal forebrain cholinergic neurons rapidly regulate cortical activity and visual perception in awake, behaving mice. Optogenetic activation of the cholinergic neurons or their V1 axon terminals improved performance of a visual discrimination task on a trial-by-trial basis. In V1, basal forebrain activation enhanced visual responses and desynchronized neuronal spiking; these changes could partly account for the behavioral improvement. Conversely, optogenetic basal forebrain inactivation decreased behavioral performance, synchronized cortical activity and impaired visual responses, indicating the importance of cholinergic activity in normal visual processing. These results underscore the causal role of basal forebrain cholinergic neurons in fast, bidirectional modulation of cortical processing and sensory perception.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Crochet, S. & Petersen, C.C.H. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat. Neurosci. 9, 608–610 (2006).
Poulet, J.F.A. & Petersen, C.C.H. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–885 (2008).
Niell, C.M. & Stryker, M.P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Castro-Alamancos, M.A. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron 41, 455–464 (2004).
Wörgötter, F. et al. State-dependent receptive-field restructuring in the visual cortex. Nature 396, 165–168 (1998).
Hasenstaub, A., Sachdev, R.N.S. & McCormick, D.A. State changes rapidly modulate cortical neuronal responsiveness. J. Neurosci. 27, 9607–9622 (2007).
Marguet, S.L. & Harris, K.D. State-dependent representation of amplitude-modulated noise stimuli in rat auditory cortex. J. Neurosci. 31, 6414–6420 (2011).
Xu, S., Jiang, W., Poo, M.-M. & Dan, Y. Activity recall in a visual cortical ensemble. Nat. Neurosci. 15, 449–455 (2012).
Buzsaki, G. et al. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J. Neurosci. 8, 4007–4026 (1988).
Zaborszky, L. & Duque, A. Sleep-wake mechanisms and basal forebrain circuitry. Front. Biosci. 8, d1146–d1169 (2003).
Jones, B.E. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol. Sci. 26, 578–586 (2005).
Lee, M.-G., Hassani, O.K., Alonso, A. & Jones, B.E. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J. Neurosci. 25, 4365–4369 (2005).
Parikh, V., Kozak, R., Martinez, V. & Sarter, M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56, 141–154 (2007).
Metherate, R. & Ashe, J.H. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse 14, 132–143 (1993).
Goard, M. & Dan, Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci. 12, 1444–1449 (2009).
Kuo, M.-C., Rasmusson, D.D. & Dringenberg, H.C. Input-selective potentiation and rebalancing of primary sensory cortex afferents by endogenous acetylcholine. Neuroscience 163, 430–441 (2009).
Zhao, S. et al. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat. Methods 8, 745–752 (2011).
Bezdudnaya, T. et al. Thalamic burst mode and inattention in the awake LGNd. Neuron 49, 421–432 (2006).
Keller, G.B., Bonhoeffer, T. & Hübener, M. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron 74, 809–815 (2012).
Polack, P.-O., Friedman, J. & Golshani, P. Cellular mechanisms of brain state–dependent gain modulation in visual cortex. Nat. Neurosci. 16, 1331–1339 (2013).
Herrero, J.L. et al. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature 454, 1110–1114 (2008).
Rokem, A., Landau, A.N., Garg, D., Prinzmetal, W. & Silver, M.A. Cholinergic enhancement increases the effects of voluntary attention but does not affect involuntary attention. Neuropsychopharmacology 35, 2538–2544 (2010).
Bauer, M. et al. Cholinergic enhancement of visual attention and neural oscillations in the human brain. Curr. Biol. 22, 397–402 (2012).
Chiba, A.A., Bushnell, P.J., Oshiro, W.M. & Gallagher, M. Selective removal of cholinergic neurons in the basal forebrain alters cued target detection. Neuroreport 10, 3119–3123 (1999).
Sarter, M., Hasselmo, M.E., Bruno, J.P. & Givens, B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Brain Res. Rev. 48, 98–111 (2005).
Sillito, A.M. & Kemp, J.A. Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res. 289, 143–155 (1983).
Laplante, F., Morin, Y., Quirion, R. & Vaucher, E. Acetylcholine release is elicited in the visual cortex, but not in the prefrontal cortex, by patterned visual stimulation: a dual in vivo microdialysis study with functional correlates in the rat brain. Neuroscience 132, 501–510 (2005).
Disney, A.A., Aoki, C. & Hawken, M.J. Gain modulation by nicotine in macaque V1. Neuron 56, 701–713 (2007).
Sato, H., Hata, Y., Masui, H. & Tsumoto, T. A functional role of cholinergic innervation to neurons in the cat visual cortex. J. Neurophysiol. 58, 765–780 (1987).
Guillem, K. et al. Nicotinic acetylcholine receptor-β2 subunits in the medial prefrontal cortex control attention. Science 333, 888–891 (2011).
Kalmbach, A., Hedrick, T. & Waters, J. Selective optogenetic stimulation of cholinergic axons in neocortex. J. Neurophysiol. 107, 2008–2019 (2012).
Tye, K.M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Warden, M.R. et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492, 428–432 (2012).
Rye, D.B., Wainer, B.H., Mesulam, M.M., Mufson, E.J. & Saper, C.B. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience 13, 627–643 (1984).
Thiele, A., Herrero, J.L., Distler, C. & Hoffmann, K.P. Contribution of cholinergic and GABAergic mechanisms to direction tuning, discriminability, response reliability, and neuronal rate correlations in macaque middle temporal area. J. Neurosci. 32, 16602–16615 (2012).
Parent, A., Pare, D., Smith, Y. & Steriade, M. Basal forebrain cholinergic and noncholinergic projections to the thalamus and brainstem in cats and monkeys. J. Comp. Neurol. 277, 281–301 (1988).
Yu, A.J. & Dayan, P. Uncertainty, neuromodulation, and attention. Neuron 46, 681–692 (2005).
Ma, M. & Luo, M. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J. Neurosci. 32, 10105–10116 (2012).
Cohen, M.R. & Maunsell, J.H.R. Attention improves performance primarily by reducing interneuronal correlations. Nat. Neurosci. 12, 1594–1600 (2009).
Reynolds, J.H., Pasternak, T. & Desimone, R. Attention increases sensitivity of V4 neurons. Neuron 26, 703–714 (2000).
Williford, T. & Maunsell, J.H.R. Effects of spatial attention on contrast response functions in macaque area V4. J. Neurophysiol. 96, 40–54 (2006).
Boynton, G.M. A framework for describing the effects of attention on visual responses. Vision Res. 49, 1129–1143 (2009).
Mitchell, J.F., Sundberg, K.A. & Reynolds, J.H. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63, 879–888 (2009).
Price, J.L. & Stern, R. Individual cells in the nucleus basalis–diagonal band complex have restricted axonal projections to the cerebral cortex in the rat. Brain Res. 269, 352–356 (1983).
Sarter, M., Parikh, V. & Howe, W.M. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat. Rev. Neurosci. 10, 383–390 (2009).
Kilgard, M.P. & Merzenich, M.M. Cortical map reorganization enabled by nucleus basalis activity. Science 279, 1714–1718 (1998).
Froemke, R.C. et al. Long-term modification of cortical synapses improves sensory perception. Nat. Neurosci. 16, 79–88 (2013).
Chubykin, A.A., Roach, E.B., Bear, M.F. & Shuler, M.G.H. A cholinergic mechanism for reward timing within primary visual cortex. Neuron 77, 723–735 (2013).
Hedrick, T. & Waters, J. Physiological properties of cholinergic and non-cholinergic magnocellular neurons in acute slices from adult mouse nucleus basalis. PLoS ONE 5, e11046 (2010).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates (Elsevier, San Diego, 2004).
Dombeck, D.A., Khabbaz, A.N., Collman, F., Adelman, T.L. & Tank, D.W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56, 43–57 (2007).
van Hateren, J.H. & van der Schaaf, A. Independent component filters of natural images compared with simple cells in primary visual cortex. Proc. R. Soc. Lond. B 265, 359–366 (1998).
Busse, L. et al. The detection of visual contrast in the behaving mouse. J. Neurosci. 31, 11351–11361 (2011).
Hazan, L., Zugaro, M. & Buzsáki, G. Klusters, NeuroScope, NDManager: a free software suite for neurophysiological data processing and visualization. J. Neurosci. Methods 155, 207–216 (2006).
Mitra, P. & Bokil, H. Observed Brain Dynamics (Oxford University Press, New York, 2008).
Britten, K.H., Shadlen, M.N., Newsome, W.T. & Movshon, J.A. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci. 12, 4745–4765 (1992).
Acknowledgements
We thank H.J. Alitto for helpful discussions, and E. Rothenberg, J. Cox, G. Pho, S. Zhang, J. Jin, S. Harding-Forrester, C. Oldfield, Y.-C. Kuo, C. Niell, M. Stryker and M. Sur for technical assistance. This work was supported by US National Institutes of Health grant R01 EY018861, National Science Foundation grant 22250400-42533 (Y.D.), National Institute of Mental Health grant RC1-MH088434 (G.F.) and a Ruth L. Kirschstein National Research Service Award (F31NS059258) from the National Institute of Neurological Disorders and Stroke (M.J.G.).
Author information
Authors and Affiliations
Contributions
L.P. and M.J.G. performed the electrophysiology experiments. L.P., M.J.G. and D.E. performed the behavioral experiments. L.P. and D.E. performed histology. M.X. and A.C.K. developed the behavioral setup. S.-H.L., T.C.H. and L.P. performed the tracing experiments. G.F. provided the ChAT-ChR2-EYFP mice. L.P. and M.J.G. analyzed the data. L.P., M.J.G. and Y.D. conceived and designed the experiments and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Targeting of basal forebrain.
(a) Schematic drawing showing stereotaxic coordinates used for targeting basal forebrain (nucleus basalis). Brain outlines adapted from ref. 50. The black square indicates approximate region shown in (b)–(d). (b) Low-magnification image showing an example of cannula lesion for a ChAT-ChR2-EYFP mouse (dashed circle) in a horizontal slice. Approximate borders of basal forebrain (nucleus basalis) and globus pallidus are outlined in white. (c) Example of a lesion for a ChAT-ARCH-GFP mouse, conventions as in (b). (d) Fiber locations relative to nucleus basalis and globus pallidus borders for 20 basal forebrain activation experiments and 10 inactivation experiments in which histology was performed after behavioral or physiology experiments. In 3 of the behavioral experiments, the fiber location was > 500 μm from the nucleus basalis border (not shown), but they were included in the experimental group in the behavioral analysis. Circles with dashed black outlines correspond to examples in (b) and (c). BF: basal forebrain, NB: nucleus basalis, GP: globus pallidus.
Supplementary Figure 2 V1-projecting cholinergic cells in the basal forebrain.
(a) Schematic coronal slice (adapted from ref. 50) with black square indicating approximate region shown in (b). (b) Low-magnification image of a ChAT-tdTomato mouse injected with the retrograde tracer CTB-488 in V1. We found V1-projecting cholinergic neurons throughout the basal forebrain, including nucleus basalis (NB), substantia innominata (SI), the horizontal limb of the diagonal band of Broca (HDB), and the magnocellular pre-optic nucleus (MCPO). These cells were found from bregma 0 to -0.8 mm in the rostrocaudal axis, 0.8 to 2 mm lateral to bregma, and 4 to 5.5 mm below the cortical surface. (c) Higher magnification images (outlined with white dashed squares in (b) showing co-localization of CTB-filled neurons (green) and ChAT neurons (red) in the NB (top) and HDB/MCPO (bottom) regions. Arrowheads indicate cells with ChAT/CTB-488 overlap. (d) Cholinergic fibers expressing EYFP in V1.
Supplementary Figure 3 Optogenetic activation of basal forebrain cholinergic neurons interacts with ongoing behavioral state.
(a) Example LFP (black) and running speed (gray) traces showing the effect of basal forebrain activation (blue bar) for representative “no run” (black) and “run” (orange) trials. Run trials were defined as the ones in which the mean speed over the 5-s window immediately preceding laser stimulation exceeded 10% of the animal's maximum speed (although the results are not sensitive to variations of this criterion). (b) LFP spectra averaged from 14 experiments with corresponding running speed averages. Blue bar: laser stimulation. Power at each frequency was normalized by baseline and color-coded (scale bar on the right). (c) Population summary of laser-induced LFP power change. Each gray circle represents the median of one experiment, black and orange circles indicate average across experiments. In “no run” trials laser stimulation caused an eight-fold reduction of low-frequency power (t(13) = −4.83, P = 3.3 × 10−4, paired t test, n = 14 mice) and no significant change at high frequencies (t(13)= −0.89, P = 0.39). In the “run” trials, laser also caused a significant reduction of low-frequency power (t(13) = −3.21, P = 0.007), although with a smaller magnitude (1.5-fold) than that in the “no run” trials (t(13) = 2.47, P = 0.03, t test). In the “run” trials laser increased the power in the high-frequency range by a factor of 20 (t(13) = 4.33, P = 9.8 × 10-4, paired t test), which is significantly different from the “no run” trials (t(13) = 4.37, P = 9.2 × 10−4). (d) Basal forebrain cholinergic activation did not change running speed (W(13) = 32, P = 0.62, Wilcoxon signed rank test). Solid line is average and shaded area indicates ± s.e.m. (n = 14 mice). Blue bar indicates laser on period. * P < 0.05, ** P < 0.01, *** P < 0.001, n.s.: not significant, BF: basal forebrain.
Supplementary Figure 4 Behavioral effect of laser stimulation on control mice.
(a) Hit and false alarm rates for wild type animals that did not express ChR2 (n = 8), with and without blue laser stimulation. Neither hit nor false alarm rates were significantly changed (Flaser(1,7) = 2.05, Plaser = 0.20; and 0.16 and 0.70, respectively, two-way repeated-measures ANOVA). (b) d' was not significantly changed by laser stimulation in control animals (Flaser(1,7) = 1.11, Plaser = 0.33). Error bars, ± s.e.m. FA: false alarm.
Supplementary Figure 5 Direct optogenetic activation of cholinergic fibers in V1 decreases low-frequency LFP power.
Top: LFP spectra averaged from experiments in 8 different mice, 30 trials/mouse. Blue bar, laser stimulation. Power at each frequency was normalized by the baseline (5-s period preceding laser onset) and color-coded (scale bar on the right). Bottom: Low-frequency power (1 – 5 Hz) is significantly decreased by direct V1 stimulation of cholinergic fibers (W(7) = 92, P = 0.01, Wilcoxon sign rank test). Interruption in axes by dashed lines indicates periods of light-induced artifact on the silicon probes. Shading, ± s.e.m.
Supplementary Figure 6 Dual retrograde tracing shows very low degrees of overlap between basal forebrain cholinergic cells projecting to different cortical areas.
(a) Representative basal forebrain images showing ChAT immunohistochemistry (top, pseudo-colored in blue), cells labeled with V1 retrobead injections (second row), cells labeled with beads injected in another cortical area (third row) and overlay (bottom). Arrow head in bottom left image indicates a cholinergic basal forebrain cell projecting to both V1 and V2 [note that the region labeled as V2 in the mouse brain atlas in fact corresponds to several functionally distinct higher visual areas (Andermann et al., 2011, Neuron 72, 1025–1039; Marshell et al., 2011, Neuron 72, 1040–1054). In these experiments we targeted the area medial and anterior to V1, roughly corresponding to area PM]. (b) Quantification of overlap between cells projecting to V1 and V2 (n = 2 mice, a total of 95 V1-projecting cells, 57 V2-projecting cells, 18 cells projecting to both), V1 and A1 (n = 5 mice, 265 V1-projecting cells, 55 A1-projecting cells, 22 to both), and V1 and medial prefrontal cortex (mPFC, n = 3 mice, 141 V1-projecting cells, 349 PFC-projecting cells, 25 to both). Shown are mean ± s.e.m. of the percentage of double labeled cells across mice. The low degrees of overlap between V1 and these areas is not due to a low efficiency of retrograde labeling, since injection of beads of both colors in V1 revealed a high degree of overlap (dashed line, n = 3 mice, 55 labeled with green beads, 71 with red beads, 55 with both).
Supplementary Figure 7 Optogenetic activation of basal forebrain (BF) cholinergic cells does not significantly affect eye movements.
(a) Distribution of eye positions for a representative mouse during the presentation of natural movie stimuli, for control trials (left) and basal forebrain activation trials (right). Each dot corresponds to a single time point. (b) Basal forebrain activation does not significantly affect eye movements during the presentation of natural movies (t(5) = −0.79, P = 0.46, paired t test, n = 6). Eye movements were quantified as the standard deviation of eye position across the recording for a given experimental condition. Gray circles and lines connect different conditions for individual mice. Bars indicate population mean, error bars: ± s.e.m. (c) Basal forebrain activation does not affect eye movements in the absence of visual stimulation (t(5) = −1.24, P = 0.27, paired t test, n = 6).
Supplementary Figure 8 Acetylcholine receptor (AChR) antagonists in V1 block most effects of basal forebrain cholinergic activation.
(a) Average LFP spectra for basal forebrain activation (blue bar on top) before (n = 8) and after (n = 8) local application of nicotinic and muscarinic AChR antagonists to V1, and after washout of the antagonists (n = 6). (b) Basal forebrain activation-induced LFP desynchronization is significantly decreased by AChR antagonists in a reversible fashion. Gray circles and lines connect different conditions for individual mice, black circles and lines indicate population mean. (c) Δ firing rate (basal forebrain on – control) averaged across contrasts before (x axis) and after (y axis) the application of AChR antagonists. The basal forebrain activation-induced increase in firing rate is significantly reduced by the drugs. Error bars, ± s.e.m. (d) The decrease in low-frequency coherence between neurons induced by basal forebrain cholinergic activation is significantly weakened by AChR antagonists. Conventions as in (c). (e) Basal forebrain activation-induced decrease in Fano factor is not significantly blocked by nicotinic and muscarinic AChR antagonists. Conventions as in (c). BF: basal forebrain, FF: Fano factor.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Movies 1 and 2 (PDF 3702 kb)
Recording from V1 in awake mice on a track ball.
Sound is from multi-unit activity recorded from one of the channels of the silicon probe. Note the responses to grating onsets and increase in firing rate when the mouse is running, as previously reported (ref. 51). (MOV 1580 kb)
Rights and permissions
About this article
Cite this article
Pinto, L., Goard, M., Estandian, D. et al. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci 16, 1857–1863 (2013). https://doi.org/10.1038/nn.3552
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3552
This article is cited by
-
Activation of M1 cholinergic receptors in mouse somatosensory cortex enhances information processing and detection behaviour
Communications Biology (2024)
-
Transcutaneous cervical vagus nerve stimulation improves sensory performance in humans: a randomized controlled crossover pilot study
Scientific Reports (2024)
-
Basal forebrain cholinergic signalling: development, connectivity and roles in cognition
Nature Reviews Neuroscience (2023)
-
A cholinergic auditory pathway
Nature Neuroscience (2023)
-
The cholinergic basal forebrain provides a parallel channel for state-dependent sensory signaling to auditory cortex
Nature Neuroscience (2023)