Abstract
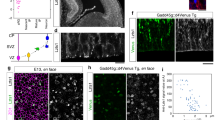
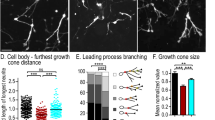
In brain development, distinct types of migration, radial migration and tangential migration, are shown by excitatory and inhibitory neurons, respectively. Whether these two types of migration operate by similar cellular mechanisms remains unclear. We examined neuronal migration in mice deficient in mDia1 (also known as Diap1) and mDia3 (also known as Diap2), which encode the Rho-regulated actin nucleators mammalian diaphanous homolog 1 (mDia1) and mDia3. mDia deficiency impaired tangential migration of cortical and olfactory inhibitory interneurons, whereas radial migration and consequent layer formation of cortical excitatory neurons were unaffected. mDia-deficient neuroblasts exhibited reduced separation of the centrosome from the nucleus and retarded nuclear translocation. Concomitantly, anterograde F-actin movement and F-actin condensation at the rear, which occur during centrosomal and nuclear movement of wild-type cells, respectively, were impaired in mDia-deficient neuroblasts. Blockade of Rho-associated protein kinase (ROCK), which regulates myosin II, also impaired nuclear translocation. These results suggest that Rho signaling via mDia and ROCK critically regulates nuclear translocation through F-actin dynamics in tangential migration, whereas this mechanism is dispensable in radial migration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ghashghaei, H.T., Lai, C. & Anton, E.S. Neuronal migration in the adult brain: are we there yet? Nat. Rev. Neurosci. 8, 141–151 (2007).
Marín, O. & Rubenstein, J.L. Cell migration in the forebrain. Annu. Rev. Neurosci. 26, 441–483 (2003).
Métin, C., Vallee, R.B., Rakic, P. & Bhide, P.G. Modes and mishaps of neuronal migration in the mammalian brain. J. Neurosci. 28, 11746–11752 (2008).
Alvarez-Buylla, A. & Garcia-Verdugo, J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 22, 629–634 (2002).
Lledo, P.M., Merkle, F.T. & Alvarez-Buylla, A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 31, 392–400 (2008).
Kawauchi, T. & Hoshino, M. Molecular pathways regulating cytoskeletal organization and morphological changes in migrating neurons. Dev. Neurosci. 30, 36–46 (2008).
Marín, O., Valdeolmillos, M. & Moya, F. Neurons in motion: same principles for different shapes? Trends Neurosci. 29, 655–661 (2006).
Xie, Z., Sanada, K., Samuels, B.A., Shih, H. & Tsai, L.H. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell 114, 469–482 (2003).
Solecki, D.J., Model, L., Gaetz, J., Kapoor, T.M. & Hatten, M.E. Par6α signaling controls glial-guided neuronal migration. Nat. Neurosci. 7, 1195–1203 (2004).
Tsai, J.W., Bremner, K.H. & Vallee, R.B. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 10, 970–979 (2007).
O'Rourke, N.A., Dailey, M.E., Smith, S.J. & McConnell, S.K. Diverse migratory pathways in the developing cerebral cortex. Science 258, 299–302 (1992).
Wichterle, H., Garcia-Verdugo, J.M. & Alvarez-Buylla, A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron 18, 779–791 (1997).
Marín, O. & Rubenstein, J.L. A long, remarkable journey: tangential migration in the telencephalon. Nat. Rev. Neurosci. 2, 780–790 (2001).
Schaar, B.T. & McConnell, S.K. Cytoskeletal coordination during neuronal migration. Proc. Natl. Acad. Sci. USA 102, 13652–13657 (2005).
Baudoin, J.P., Alvarez, C., Gaspar, P. & Metin, C. Nocodazole-induced changes in microtubule dynamics impair the morphology and directionality of migrating medial ganglionic eminence cells. Dev. Neurosci. 30, 132–143 (2008).
Martini, F.J. & Valdeolmillos, M. Actomyosin contraction at the cell rear drives nuclear translocation in migrating cortical interneurons. J. Neurosci. 30, 8660–8670 (2010).
Bellion, A., Baudoin, J.P., Alvarez, C., Bornens, M. & Metin, C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 25, 5691–5699 (2005).
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Govek, E.E., Newey, S.E. & Van Aelst, L. The role of the Rho GTPases in neuronal development. Genes Dev. 19, 1–49 (2005).
Nguyen, L., Besson, A., Roberts, J.M. & Guillemot, F. Coupling cell cycle exit, neuronal differentiation and migration in cortical neurogenesis. Cell Cycle 5, 2314–2318 (2006).
Govek, E.E., Hatten, M.E. & Van Aelst, L. The role of Rho GTPase proteins in CNS neuronal migration. Dev. Neurobiol. 71, 528–553 (2011).
Narumiya, S., Ishizaki, T. & Watanabe, N. Rho effectors and reorganization of actin cytoskeleton. FEBS Lett. 410, 68–72 (1997).
Kimura, K. et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, 245–248 (1996).
Ishizaki, T. et al. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 404, 118–124 (1997).
Watanabe, N., Kato, T., Fujita, A., Ishizaki, T. & Narumiya, S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1, 136–143 (1999).
Higashida, C. et al. Actin polymerization–driven molecular movement of mDia1 in living cells. Science 303, 2007–2010 (2004).
Campellone, K.G. & Welch, M.D. A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 11, 237–251 (2010).
Konno, D. et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 10, 93–101 (2008).
Wen, Y. et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 6, 820–830 (2004).
Ishizaki, T. et al. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat. Cell Biol. 3, 8–14 (2001).
Palazzo, A.F., Cook, T.A., Alberts, A.S. & Gundersen, G.G. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3, 723–729 (2001).
Westermann, S. & Weber, K. Post-translational modifications regulate microtubule function. Nat. Rev. Mol. Cell Biol. 4, 938–948 (2003).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 (2008).
Vaughan, S. & Dawe, H.R. Common themes in centriole and centrosome movements. Trends Cell Biol. 21, 57–66 (2011).
Rosenblatt, J., Cramer, L.P., Baum, B. & McGee, K.M. Myosin II–dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell 117, 361–372 (2004).
Cao, J., Crest, J., Fasulo, B. & Sullivan, W. Cortical Actin dynamics facilitate early-stage centrosome separation. Curr. Biol. 20, 770–776 (2010).
Nguyen, L. et al. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 20, 1511–1524 (2006).
Ge, W. et al. Coupling of cell migration with neurogenesis by proneural bHLH factors. Proc. Natl. Acad. Sci. USA 103, 1319–1324 (2006).
Pacary, E. et al. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling. Neuron 69, 1069–1084 (2011).
Patel, B.N. & Van Vactor, D.L. Axon guidance: the cytoplasmic tail. Curr. Opin. Cell Biol. 14, 221–229 (2002).
Sawamoto, K. et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 311, 629–632 (2006).
Sakata, D. et al. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J. Exp. Med. 204, 2031–2038 (2007).
Yagi, T. et al. A novel ES cell line, TT2, with high germline-differentiating potency. Anal. Biochem. 214, 70–76 (1993).
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991).
Yasuda, S. et al. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature 428, 767–771 (2004).
Watanabe, N. et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 16, 3044–3056 (1997).
Watanabe, S. et al. mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol. Biol. Cell 19, 2328–2338 (2008).
Masahira, N. et al. Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Dev. Biol. 293, 358–369 (2006).
Saito, T. & Nakatsuji, N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 240, 237–246 (2001).
Umeshima, H., Hirano, T. & Kengaku, M. Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc. Natl. Acad. Sci. USA 104, 16182–16187 (2007).
Acknowledgements
We thank M. Okabe for providing pCX-EGFP, F. Matsuzaki for providing pCAG-EGFP-C1 and pCAG-PACT-mKO1, Y. Yanagawa for providing GAD65 and GAD67 antisense probes, Y. Deguchi for providing mDia3 riboprobes, A. Mizutani for animal care, K. Tohyama for supporting genotyping and plasmid construction, T. Arai and A. Washimi for secretarial help, and K. Nonomura for technical assistance. R.S. thanks A. Kakizuka for providing the opportunity to work in Kyoto University Graduate School of Medicine. R.S. was supported by the Japan Society for the Promotion of Science Research Fellowship. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a Core Research for Evolutional Science and Technology (CREST) grant from Japan Science and Technology Agency.
Author information
Authors and Affiliations
Contributions
R.S., D.T., T.F. and S.N. designed the study. R.S. performed most of the experiments. H. Kamijo, R.S., H. Kiyonari, T.I. and S.N. generated mDia3 knockout and mDia DKO mice. D.T. and R.S. carried out in utero electroporation for live imaging. R.S. collected and analyzed the imaging data. N.K. and K.S. performed the whole-mount immunostaining. K.W. and H.T. provided essential advices in setting up and performing in utero electroporation, and conducted in situ hybridization. S.N. and T.F. supervised the project. R.S., T.F. and S.N. wrote the manuscript. All authors discussed the results and concurred on the contents of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 (PDF 7632 kb)
Supplementary Video 1
Time-lapse, phase-contrast images of neuroblast migration from a wild-type SVZ explant in Matrigel. Images were acquired at 5 min intervals for 8 h. (MOV 2141 kb)
Supplementary Video 2
Time-lapse, phase-contrast images of neuroblast migration from an mDia-DKO SVZ explant in Matrigel. Images were acquired at 5 min intervals for 8 h. (MOV 1343 kb)
Supplementary Video 3
Time-lapse fluorescent images of migrating SVZ neuroblasts expressing EGFP from a wild-type explant in Matrigel. Images were acquired at 3 min intervals for 45 min. (MOV 340 kb)
Supplementary Video 4
Time-lapse fluorescent images of migrating SVZ neuroblasts expressing EGFP from an mDia-DKO explant in Matrigel. Images were acquired at 3 min intervals for 45 min. (MOV 940 kb)
Supplementary Video 5
Time-lapse fluorescent images of a wild-type SVZ neuroblast during the nuclear translocation in Matrigel. Images were acquired at 3 min intervals for 15 min. Signals of EGFP and PACT-mKO1 are shown in green and purple, respectively. (MOV 566 kb)
Supplementary Video 6
Time-lapse fluorescent images of an mDia-DKO SVZ neuroblast during the nuclear translocation in Matrigel. Images were acquired at 3 min intervals for 33 min. Signals of EGFP and PACT-mKO1 are shown in green and purple, respectively. (MOV 783 kb)
Supplementary Video 7
Time-lapse fluorescent images of F-actin dynamics during the nuclear translocation in two representative wild-type SVZ neuroblasts. Images were acquired at 3 min intervals for 21 or 18 min for respective neuroblasts. Signals of Lifeact-EGFP and PACT-mKO1 are shown in green and purple, respectively. (MOV 1459 kb)
Supplementary Video 8
Time-lapse fluorescent images of F-actin dynamics during the nuclear translocation in two representative mDia-DKO SVZ neuroblasts. Images were acquired at 3 min intervals for 15 or 33 min for respective neuroblasts. Signals of Lifeact-EGFP and PACT-mKO1 are shown in green and purple, respectively. (MOV 1285 kb)
Supplementary Video 9
Time-lapse fluorescent images of a migrating SVZ neuroblast without Y-27632, a ROCK inhibitor, in Matrigel. Images were acquired at 3 min intervals for 36 min. Signals of EGFP and PACT-mKO1 are shown in green and purple, respectively. (MOV 1154 kb)
Supplementary Video 10
Time-lapse fluorescent images of a migrating SVZ neuroblast treated with Y-27632, a ROCK inhibitor, in Matrigel. Images were acquired at 3 min intervals for 30 min. Signals of EGFP and PACT-mKO1 are shown in green and purple, respectively. (MOV 2617 kb)
Rights and permissions
About this article
Cite this article
Shinohara, R., Thumkeo, D., Kamijo, H. et al. A role for mDia, a Rho-regulated actin nucleator, in tangential migration of interneuron precursors. Nat Neurosci 15, 373–380 (2012). https://doi.org/10.1038/nn.3020
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3020
This article is cited by
-
Diaphanous-related formin mDia2 regulates beta2 integrins to control hematopoietic stem and progenitor cell engraftment
Nature Communications (2020)
-
Quantitative high-precision imaging of myosin-dependent filamentous actin dynamics
Journal of Muscle Research and Cell Motility (2020)
-
Olfactory memory is enhanced in mice exposed to extremely low-frequency electromagnetic fields via Wnt/β-catenin dependent modulation of subventricular zone neurogenesis
Scientific Reports (2018)
-
Hemopexin is required for adult neurogenesis in the subventricular zone/olfactory bulb pathway
Cell Death & Disease (2018)
-
Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice
Molecular Psychiatry (2018)