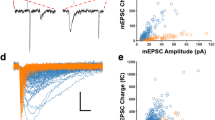
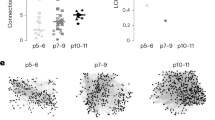
Abstract
Feedforward GABAergic inhibition sets the dendritic integration window, thereby controlling timing and output in cortical circuits. However, the manner in which feedforward inhibitory circuits emerge is unclear, despite this being a critical step for neocortical development and function. We found that sensory experience drove plasticity of the feedforward inhibitory circuit in mouse layer 4 somatosensory barrel cortex in the second postnatal week via two distinct mechanisms. First, sensory experience selectively strengthened thalamocortical-to-feedforward interneuron inputs via a presynaptic mechanism but did not regulate other inhibitory circuit components. Second, experience drove a postsynaptic mechanism in which a downregulation of a prominent thalamocortical NMDA excitatory postsynaptic potential in stellate cells regulated the final expression of functional feedforward inhibitory input. Thus, experience is required for specific, coordinated changes at thalamocortical synapses onto both inhibitory and excitatory neurons, producing a circuit plasticity that results in maturation of functional feedforward inhibition in layer 4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Woolsey, T.A. & Van der Loos, H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 17, 205–242 (1970).
Bureau, I., von Saint Paul, F. & Svoboda, K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 4, e382 (2006).
Petreanu, L., Mao, T., Sternson, S.M. & Svoboda, K. The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145 (2009).
Porter, J.T., Johnson, C.K. & Agmon, A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J. Neurosci. 21, 2699–2710 (2001).
Swadlow, H.A. Thalamocortical control of feed-forward inhibition in awake somatosensory 'barrel' cortex. Phil. Trans. R. Soc. Lond. B 357, 1717–1727 (2002).
Gabernet, L., Jadhav, S.P., Feldman, D.E., Carandini, M. & Scanziani, M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48, 315–327 (2005).
Daw, M.I., Ashby, M.C. & Isaac, J.T. Coordinated developmental recruitment of latent fast spiking interneurons in layer IV barrel cortex. Nat. Neurosci. 10, 453–461 (2007).
Cruikshank, S.J., Lewis, T.J. & Connors, B.W. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat. Neurosci. 10, 462–468 (2007).
Sun, Q.Q., Huguenard, J.R. & Prince, D.A. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J. Neurosci. 26, 1219–1230 (2006).
Bruno, R.M. & Sakmann, B. Cortex is driven by weak, but synchronously active, thalamocortical synapses. Science 312, 1622–1627 (2006).
Kremkow, J., Perrinet, L.U., Masson, G.S. & Aertsen, A. Functional consequences of correlated excitatory and inhibitory conductances in cortical networks. J. Comput. Neurosci. 28, 579–594 (2010).
Wang, H.P., Spencer, D., Fellous, J.M. & Sejnowski, T.J. Synchrony of thalamocortical inputs maximizes cortical reliability. Science 328, 106–109 (2010).
Fox, K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience 111, 799–814 (2002).
Foeller, E. & Feldman, D.E. Synaptic basis for developmental plasticity in somatosensory cortex. Curr. Opin. Neurobiol. 14, 89–95 (2004).
Fox, K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J. Neurosci. 12, 1826–1838 (1992).
Shoykhet, M., Land, P.W. & Simons, D.J. Whisker trimming begun at birth or on postnatal day 12 affects excitatory and inhibitory receptive fields of layer IV barrel neurons. J. Neurophysiol. 94, 3987–3995 (2005).
Lee, S.H., Land, P.W. & Simons, D.J. Layer- and cell type–specific effects of neonatal whisker-trimming in adult rat barrel cortex. J. Neurophysiol. 97, 4380–4385 (2007).
Simons, D.J. & Land, P.W. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature 326, 694–697 (1987).
Stern, E.A., Maravall, M. & Svoboda, K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron 31, 305–315 (2001).
Hull, C., Isaacson, J.S. & Scanziani, M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J. Neurosci. 29, 9127–9136 (2009).
Maffei, A., Nataraj, K., Nelson, S.B. & Turrigiano, G.G. Potentiation of cortical inhibition by visual deprivation. Nature 443, 81–84 (2006).
Sun, Q.Q. Experience-dependent intrinsic plasticity in interneurons of barrel cortex layer IV. J. Neurophysiol. 102, 2955–2973 (2009).
Allen, C.B., Celikel, T. & Feldman, D.E. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat. Neurosci. 6, 291–299 (2003).
Hardingham, N., Wright, N., Dachtler, J. & Fox, K. Sensory deprivation unmasks a PKA-dependent synaptic plasticity mechanism that operates in parallel with CaMKII. Neuron 60, 861–874 (2008).
Heynen, A.J. et al. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat. Neurosci. 6, 854–862 (2003).
Yoon, B.J., Smith, G.B., Heynen, A.J., Neve, R.L. & Bear, M.F. Essential role for a long-term depression mechanism in ocular dominance plasticity. Proc. Natl. Acad. Sci. USA 106, 9860–9865 (2009).
Kullmann, D.M. & Lamsa, K.P. Long-term synaptic plasticity in hippocampal interneurons. Nat. Rev. Neurosci. 8, 687–699 (2007).
Oren, I., Nissen, W., Kullmann, D.M., Somogyi, P. & Lamsa, K.P. Role of ionotropic glutamate receptors in long-term potentiation in rat hippocampal CA1 oriens–lacunosum moleculare interneurons. J. Neurosci. 29, 939–950 (2009).
Mierau, S.B., Meredith, R.M., Upton, A.L. & Paulsen, O. Dissociation of experience-dependent and -independent changes in excitatory synaptic transmission during development of barrel cortex. Proc. Natl. Acad. Sci. USA 101, 15518–15523 (2004).
Philpot, B.D., Sekhar, A.K., Shouval, H.Z. & Bear, M.F. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 29, 157–169 (2001).
Franks, K.M. & Isaacson, J.S. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron 47, 101–114 (2005).
Binshtok, A.M., Fleidervish, I.A., Sprengel, R. & Gutnick, M.J. NMDA receptors in layer 4 spiny stellate cells of the mouse barrel cortex contain the NR2C subunit. J. Neurosci. 26, 708–715 (2006).
Espinosa, F. & Kavalali, E.T. NMDA receptor activation by spontaneous glutamatergic neurotransmission. J. Neurophysiol. 101, 2290–2296 (2009).
Das, S. et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 393, 377–381 (1998).
Al-Hallaq, R.A. et al. Association of NR3A with the N-methyl-D-aspartate receptor NR1 and NR2 subunits. Mol. Pharmacol. 62, 1119–1127 (2002).
Wong, H.K. et al. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J. Comp. Neurol. 450, 303–317 (2002).
Monyer, H., Burnashev, N., Laurie, D.J., Sakmann, B. & Seeburg, P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540 (1994).
Morishita, W., Marie, H. & Malenka, R.C. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat. Neurosci. 8, 1043–1050 (2005).
Bellone, C. & Nicoll, R.A. Rapid bidirectional switching of synaptic NMDA receptors. Neuron 55, 779–785 (2007).
Feldmeyer, D., Egger, V., Lubke, J. & Sakmann, B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single 'barrel' of developing rat somatosensory cortex. J. Physiol. (Lond.) 521, 169–190 (1999).
Petersen, C.C. & Sakmann, B. The excitatory neuronal network of rat layer 4 barrel cortex. J. Neurosci. 20, 7579–7586 (2000).
Lefort, S., Tomm, C., Floyd Sarria, J.C. & Petersen, C.C. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61, 301–316 (2009).
Tamamaki, N. et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 467, 60–79 (2003).
Isaac, J.T., Crair, M.C., Nicoll, R.A. & Malenka, R.C. Silent synapses during development of thalamocortical inputs. Neuron 18, 269–280 (1997).
Agmon, A. & Connors, B.W. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41, 365–379 (1991).
Isaac, J.T., Oliet, S.H., Hjelmstad, G.O., Nicoll, R.A. & Malenka, R.C. Expression mechanisms of long-term potentiation in the hippocampus. J. Physiol. (Paris) 90, 299–303 (1996).
Acknowledgements
We thank C. McBain for discussions and Y. Yanagawa (Gunma University) for providing GAD67-GFP knockin mouse. This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Program.
Author information
Authors and Affiliations
Contributions
R.C. performed and analyzed the experiments. R.C. and J.T.R.I. designed the experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 237 kb)
Rights and permissions
About this article
Cite this article
Chittajallu, R., Isaac, J. Emergence of cortical inhibition by coordinated sensory-driven plasticity at distinct synaptic loci. Nat Neurosci 13, 1240–1248 (2010). https://doi.org/10.1038/nn.2639
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2639
This article is cited by
-
Neuropathological signatures revealed by transcriptomic and proteomic analysis in Pten-deficient mouse models
Scientific Reports (2023)
-
Step by step: cells with multiple functions in cortical circuit assembly
Nature Reviews Neuroscience (2022)
-
(−)-Gallocatechin gallate from green tea rescues cognitive impairment through restoring hippocampal silent synapses in post-menopausal depression
Scientific Reports (2021)
-
Cellular and synaptic phenotypes lead to disrupted information processing in Fmr1-KO mouse layer 4 barrel cortex
Nature Communications (2019)
-
Neurogliaform cells dynamically regulate somatosensory integration via synapse-specific modulation
Nature Neuroscience (2013)