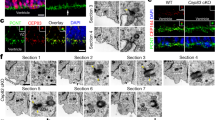
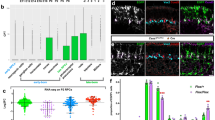
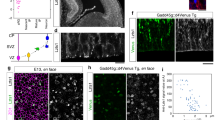
Abstract
The mechanisms that regulate symmetric, proliferative divisions versus asymmetric, neurogenic divisions of mammalian neural precursors are still not well understood. We found that Lfc (Arhgef2), a Rho-specific guanine nucleotide exchange factor that interacts with spindle microtubules, and its negative regulator Tctex-1 (Dynlt1) determine the genesis of neurons from precursors in the embryonic murine cortex. Specifically, genetic knockdown of Arhgef2 in cortical precursors either in culture or in vivo inhibited neurogenesis and maintained cells as cycling radial precursors. Conversely, genetic knockdown of Dynlt1 in radial precursors promoted neurogenesis and depleted cycling cortical precursors. Coincident silencing of these two genes indicated that Tctex-1 normally inhibits the genesis of neurons from radial precursors by antagonizing the proneurogenic actions of Lfc. Moreover, Lfc and Tctex-1 were required to determine the orientation of mitotic precursor cell divisions in vivo. Thus, Lfc and Tctex-1 interact to regulate cortical neurogenesis, potentially by regulating mitotic spindle orientation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Götz, M. & Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788 (2005).
Zhong, W. & Chia, W. Neurogenesis and asymmetric cell division. Curr. Opin. Neurobiol. 18, 4–11 (2008).
Noctor, S.C., Martinez-Cerdeno, V., Ivic, L. & Kriegstein, A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136–144 (2004).
Gal, J.S. et al. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J. Neurosci. 26, 1045–1056 (2006).
Attardo, A., Calegari, F., Haubensak, W., Wilsch-Brauninger, M. & Huttner, W.B. Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS One 3, e2388 (2008).
Haydar, T.F., Ang, E., Jr. & Rakic, P. Mitotic spindle rotation and mode of cell division in the developing telecephalon. Proc. Natl. Acad. Sci. USA 100, 2890–2895 (2003).
Bonfanti, L. & Peretto, P. Radial glial origin of the adult neural stem cells in the subventricular zone. Prog. Neurobiol. 83, 24–36 (2007).
Guillemot, F. Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr. Opin. Cell Biol. 17, 639–647 (2005).
Miller, F.D. & Gauthier, A.S. Timing is everything: making neurons versus glial in the developing cortex. Neuron 54, 357–369 (2007).
Gönczy, P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 9, 355–366 (2008).
Farkas, L.M. & Huttner, W.B. The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr. Opin. Cell Biol. 20, 707–715 (2008).
Benais-Pont, G. et al. Identification of a tight junction–associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J. Cell Biol. 160, 729–740 (2003).
Bakal, C.J. et al. The Rho GTP exchange factor Lfc promotes spindle assembly in early mitosis. Proc. Natl. Acad. Sci. USA 102, 9529–9534 (2005).
Glaven, J.A., Whitehead, I.P., Nomanbhoy, T., Kay, R. & Cerione, R.A. Lfc and Lsc oncoproteins represent two new guanine nucleotide exchange factors for the Rho GTP-binding protein. J. Biol. Chem. 271, 27374–27381 (1996).
Glaven, J.A., Whitehead, I., Bagrodia, S., Kay, R. & Cerione, R.A. The dbl-related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J. Biol. Chem. 274, 2279–2285 (1999).
Krendel, M., Zenke, F.T. & Bokoch, G.M. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 4, 294–301 (2002).
Dedesma, C., Chuang, J.-Z., Alfinito, P.D. & Sung, C.-H. Dynein light chain Tctex-1 identifies neural progenitors in adult brain. J. Comp. Neurol. 496, 773–786 (2006).
King, S.M. et al. The mouse t-complex–encoded protein Tctex-1 is a light chain of brain cytoplasmic dynein. J. Biol. Chem. 271, 32281–32287 (1996).
Sachdev, P. et al. G protein βγ subunit interaction with the dynein light-chain component tctex-1 regulates neurite outgrowth. EMBO J. 26, 2621–2632 (2007).
Yoshizawa, M. et al. Dynamic and coordinated expression profile of dbl-family guanine nucleotide exchange factors in the developing mouse brain. Gene Expr. Patterns 3, 375–381 (2003).
Barnabé-Heider, F. et al. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 48, 253–265 (2005).
Gauthier, A.S. et al. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron 54, 245–262 (2007).
Feng, L., Hatter, M.E. & Heintz, N. Brain lipid-binding protein (BLBP): a novel signlaling system in the developing mammalian CNS. Neuron 12, 895–908 (1994).
Götz, M., Stoykova, A. & Gruss, P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 21, 1031–1044 (1998).
Ryan, X.P. et al. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron 47, 85–100 (2005).
Haubensak, W., Attardo, A., Denk, W. & Huttner, B. Neurons arise in the basal neuroepithelium of the early mammalian telecephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. USA 101, 3196–3201 (2004).
Chuang, J.Z., Milner, T.A. & Sung, C.H. Subunit heterogeneity of cytoplasmic dynein: differential expression of 14 kDa dynein light chains in rat hippocampus. J. Neurosci. 21, 5501–5512 (2001).
Chuang, J.Z., Yeh, T.Y., Bollati., F., Conde, C., Canavosio, F., Caceres, A. & Sung, C.H. The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev. Cell 9, 75–86 (2005).
Paquin, A., Barnabé-Heider, F., Kageyama, R. & Miller, F.D. CCAAT/enhancer-binding protein phosphorylation biases cortical precursors to generate neurons rather than astrocytes in vivo. J. Neurosci. 25, 10747–10758 (2005).
Tai, A.W., Chuang, J.Z. & Sung, C.H. Localization of Tctex-1, a cytoplasmic dynein light chain, to the Golgi apparatus and evidence for dynein complex heterogeneity. J. Biol. Chem. 273, 19639–19649 (1998).
Fish, J.L., Kosodo, Y., Enard, W., Paabo, S. & Huttner, W.B. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc. Natl. Acad. Sci. USA 103, 10438–10443 (2006).
Fish, J.L., Dehay, C., Kennedy, H. & Huttner, W.B. Making bigger brains—the evolution of neural progenitor–cell division. J. Cell Sci. 121, 2783–2793 (2008).
Kosodo, Y. et al. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 23, 2314–2324 (2004).
Olenik, C., Aktories, K. & Meyer, D.K. Differential expression of the small GTP-binding proteins RhoA, RhoB, Cdc42u and Cdc42b in developing rat neocortex. Brain Res. Mol. Brain Res. 70, 9–17 (1999).
Narumiya, S. & Yasuda, S. Rho GTPases in animal cell mitosis. Curr. Opin. Cell Biol. 18, 199–205 (2006).
Cappello, S. et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat. Neurosci. 9, 1099–1107 (2006).
Roszko, I., Alfonso, C., Henrique, D. & Mathis, L. Key role played by RhoA in the balance between planar and apico-basal cell divisions in the chick neuroepithelium. Dev. Biol. 298, 212–224 (2006).
Aijaz, S., D'Atri, F., Citi, S., Balda, M.S. & Matter, K. Binding of GEF-H1 to the tight junction–associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev. Cell 8, 777–786 (2005).
Tai, A.W., Chuang, J.Z., Bode, C., Wolfrum, U. & Sung, C. H. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell 97, 877–887 (1999).
Lanier, S.M. AGS proteins, GPR motifs and the signals processed by heterotrimeric G proteins. Biol. Cell 96, 369–372 (2004).
Sanada, K.S. & Tsai, L.H. G protein βγ subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell 122, 119–131 (2005).
Kholmanskikh, S.S., Dobrin, J.S., Wynshaw-Boris, A., Letourneau, P.C. & Ross, M.E. Disregulated RhoGTPases and actin cytoskeleton contribute to the migration defect in Lis-1–deficient neurons. J. Neurosci. 23, 8673–8681 (2003).
Nguyen, L. et al. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 305, 187–202 (2001).
Piao, X. et al. G protein–coupled receptor–dependent development of human frontal cortex. Science 303, 2033–2036 (2004).
Estivill-Torrús, G. et al. Absence of LPA1 signaling results in defective cortical development. Cereb. Cortex 18, 938–950 (2008).
Yano, H. et al. Association of Trk neurotrophin receptors with components of the cytoplasmic dynein motor. J. Neurosci. 21, RC125 (2001).
Bartkowska, K., Paquin, A., Gauthier, A.S., Kaplan, D.R. & Miller, F.D. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development 134, 4369–4380 (2007).
Machado, R.D. et al. C. Functional interaction between BMPR-II and tctex-1, a light chain of dynein, is isoform-specific and disrupted by mutations underlying primary pulmonary hypertension. Hum. Mol. Genet. 12, 3277–3286 (2003).
Li, W., Cogswell, C.A. & LoTurco, J.J. Neuronal differentiation of precursors in the neocortical ventricular zone is triggered by BMP. J. Neurosci. 18, 8853–8862 (1998).
Gross, R.E. et al. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17, 595–606 (1996).
Acknowledgements
We thank C.H. Sung for his generous gift of the Tctex-1 antibody. We thank J. Biernaskie, K. Park, A. Paquin, S. Dugani, M. Fujitani, N. Fine and J. LaRose for their advice and assistance. This work was supported by a grant from the Canadian Institutes of Health Research (MOP-13958) to F.D.M. and D.R.K. F.D.M. and D.R.K. are Senior Canada Research Chairs and F.D.M. is a Howard Hughes Medical Institute International Research Scholar. A.G.-F. was supported by a studentship from the Natural Sciences and Engineering Research Council of Canada and by a Hospital for Sick Children Foundation studentship award. D.C.L. was supported by a post-doctoral fellowship from the Heart and Stroke Foundation of Canada.
Author information
Authors and Affiliations
Contributions
A.G.-F. performed all of the in utero electroporations and in vivo analyses, the culture rescue experiments, the clonal analyses, the localization of Tctex-1 and co-wrote the paper. A.G.-F. contributed 60 data panels and A.G.-F. and/or D.C.L. contributed the remainder. D.C.L. prepared constructs, performed the western blots, analyzed Lfc expression in culture and co-wrote the paper. D.C.L. and A.G.-F. together carried out the culture analyses and the mitotic spindle counts. M.G. generated or provided constructs and antibodies. D.R.K., R.R. and F.D.M. all contributed intellectual guidance. D.R.K. and F.D.M. co-supervised all of the experiments. R.R. generated the initial hypothesis and co-supervised the biochemical experiments. F.D.M. co-wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 1677 kb)
Rights and permissions
About this article
Cite this article
Gauthier-Fisher, A., Lin, D., Greeve, M. et al. Lfc and Tctex-1 regulate the genesis of neurons from cortical precursor cells. Nat Neurosci 12, 735–744 (2009). https://doi.org/10.1038/nn.2339
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2339
This article is cited by
-
Comprehensive ceRNA network for MACF1 regulates osteoblast proliferation
BMC Genomics (2022)
-
Activation of neural lineage networks and ARHGEF2 in enzalutamide-resistant and neuroendocrine prostate cancer and association with patient outcomes
Communications Medicine (2022)
-
Altered cleavage plane orientation with increased genomic aneuploidy produced by receptor-mediated lysophosphatidic acid (LPA) signaling in mouse cerebral cortical neural progenitor cells
Molecular Brain (2020)
-
Progenitor genealogy in the developing cerebral cortex
Cell and Tissue Research (2015)
-
Rnd3 coordinates early steps of cortical neurogenesis through actin-dependent and -independent mechanisms
Nature Communications (2013)