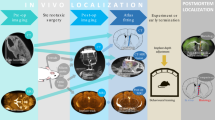
Abstract
The localization of microelectrode recording sites in the layers of primate cerebral cortex permits the analysis of relationships between recorded neuronal activities and underlying anatomical connections. We present a magnetic resonance imaging method for precise in vivo localization of cortical recording sites. In this method, the susceptibility-induced effect thickens the appearance of the microelectrode and enhances the detectability of the microelectrode tip, which usually occupies less than a few percent of the volume of an image voxel. In a phantom study, the optimized susceptibility-induced effect allowed tip detection with single-voxel accuracy (in-plane resolution, 50 μm). We applied this method to recording microelectrodes inserted into the brains of macaque monkeys, and localized the microelectrode tip at an in-plane resolution of 150 μm within the cortex of 2–3 mm in thickness. Subsequent histological analyses validated the single-voxel accuracy of the in vivo tip localization. This method opens up a way to investigate information flow during cognitive processes in the brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hubel, D.H. & Wiesel, T.N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. (Lond.) 160, 106–154 (1962).
Evarts, E.V. A technique for recording activity of subcortical neurons in moving animals. Electroencephalog. Clin. Neurophysiol. 24, 83–86 (1968).
Felleman, D.J. & Van Essen, D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991).
Andersen, R.A. & Buneo, C.A. Intentional maps in posterior parietal cortex. Annu. Rev. Neurosci. 25, 189–220 (2002).
Nakahara, K., Hayashi, T., Konishi, S. & Miyashita, Y. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science 295, 1532–1536 (2002).
Douglas, R., Markram, H. & Martin, K. Neocortex. in The Synaptic Organization of the Brain 5th edn. (ed. Shepherd, G.M.) 499–558 (Oxford University Press, New York, 2004).
Suzuki, H. & Azuma, M. A glass-insulated “Elgiloy” microelectrode for recording unit activity in chronic monkey experiments. Electroencephalogr. Clin. Neurophysiol. 41, 93–95 (1976).
Nahm, F.K., Dale, A.M., Albright, T.D. & Amaral, D.G. In vivo microelectrode localization in the brain of the alert monkey: a combined radiographic and magnetic resonance imaging approach. Exp. Brain Res. 98, 401–411 (1994).
Fung, S.H., Burstein, D. & Born, R.T. In vivo microelectrode track reconstruction using magnetic resonance imaging. J. Neurosci. Methods 80, 215–224 (1998).
Glimcher, P.W. et al. Application of neurosonography to experimental physiology. J. Neurosci. Methods 108, 131–144 (2001).
Jog, M.S. et al. Tetrode technology: advances in implantable hardware, neuroimaging, and data analysis techniques. J. Neurosci. Methods 117, 141–152 (2002).
Tammer, R. et al. Compatibility of glass-guided recording microelectrodes in the brain stem of squirrel monkeys with high-resolution 3D MRI. J. Neurosci. Methods 153, 221–229 (2006).
Haacke, E.M., Brown, R.W., Thompson, M.R. & Venkatesan, R. Magnetic Resonance Imaging: Physical Principles and Sequence Design (John Wiley & Sons, New York, 1999).
Aube, C. et al. Magnetic resonance imaging characteristics of six radiofrequency electrodes in a phantom study. J. Vasc. Interv. Radiol. 15, 385–392 (2004).
Bezdek, J.C., Hall, L.O. & Clarke, L.P. Review of MR image segmentation techniques using pattern recognition. Med. Phys. 20, 1033–1048 (1993).
Barbier, E.L. et al. Imaging cortical anatomy by high-resolution MR at 3.0T: detection of the stripe of Gennari in visual area 17. Magn. Reson. Med. 48, 735–738 (2002).
Fatterpekar, G.M. et al. Cytoarchitecture of the human cerebral cortex: MR microscopy of excised specimens at 9.4 Tesla. AJNR Am. J. Neuroradiol. 23, 1313–1321 (2002).
Pfeuffer, J., Merkle, H., Beyerlein, M., Steudel, T. & Logothetis, N.K. Anatomical and functional MR imaging in the macaque monkey using a vertical large-bore 7 Tesla setup. Magn. Reson. Imaging 22, 1343–1359 (2004).
Eickhoff, S. et al. High-resolution MRI reflects myeloarchitecture and cytoarchitecture of human cerebral cortex. Hum. Brain Mapp. 24, 206–215 (2005).
Koyama, M. et al. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron 41, 795–807 (2004).
Uitti, R.J. et al. Magnetic resonance imaging and deep brain stimulation. Neurosurgery 6, 1423–1428 (2002).
Martin, A.J. et al. Placement of deep brain stimulator electrodes using real-time high-field interventional magnetic resonance imaging. Magn. Reson. Med. 54, 1107–1114 (2005).
Hall, W.A., Galichich, W., Bergman, T. & Truwit, C.L. 3-Tesla intraoperative MR imaging for neurosurgery. J. Neurooncol. 77, 297–303 (2006).
Susil, R.C. et al. Transrectal prostate biopsy and fiducial marker placement in a standard 1.5T magnetic resonance imaging scanner. J. Urol. 175, 113–120 (2006).
Logothetis, N., Merkle, H., Augath, M., Trinath, T. & Ugurbil, K. Ultra high-resolution fMRI in monkeys with implanted RF coils. Neuron 35, 227–242 (2002).
Lewis, J.W. & Van Essen, D.C. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J. Comp. Neurol. 428, 79–111 (2000).
Wessberg, J. et al. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature 408, 361–365 (2000).
Andersen, R.A., Mesallam, S. & Pesaran, B. Selecting the signals for a brain-machine interface. Curr. Opin. Neurobiol. 14, 720–726 (2004).
Schuirmann, D.J. A comparison of two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J. Pharmacokinet. Biopharm. 15, 657–680 (1987).
Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 (1979).
Acknowledgements
We thank T. Watanabe for technical assistance and M. Kinoshita for helpful comments on the development of the nonmagnetic mini-manipulator. This work was supported by a Grant-in-Aid for Specially Promoted Research from Ministry for Education, Culture, Sports, Science and Technology (MEXT) to Y.M (14002005), a Grant-in-Aid for Scientific Research from MEXT to K.N. (17500202) and Y.N. (15500206), and Japan Society for the Promotion of Science Research Fellowships for Young Scientists to K.W.K (1711962) and M.K. (1511804) and by Takeda Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
A non-magnetic microdrive mini-manipulator for microelectrode implantation.
Supplementary Table 1
Scan parameters for magnetic resonance imaging of the phantom in vitro.
Supplementary Table 2
Scan parameters for magnetic resonance imaging of the monkeys in vivo.
Supplementary Table 3
Scan parameters for in vivo stability test of microelectrode position across days.
Rights and permissions
About this article
Cite this article
Matsui, T., Koyano, K., Koyama, M. et al. MRI-based localization of electrophysiological recording sites within the cerebral cortex at single-voxel accuracy. Nat Methods 4, 161–168 (2007). https://doi.org/10.1038/nmeth987
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth987
This article is cited by
-
Perirhinal circuits for memory processing
Nature Reviews Neuroscience (2019)
-
Dynamic laminar rerouting of inter-areal mnemonic signal by cognitive operations in primate temporal cortex
Nature Communications (2018)
-
Image-based in vivo assessment of targeting accuracy of stereotactic brain surgery in experimental rodent models
Scientific Reports (2016)
-
Associative-memory representations emerge as shared spatial patterns of theta activity spanning the primate temporal cortex
Nature Communications (2016)
-
Setup and data analysis for functional magnetic resonance imaging of awake cat visual cortex
Neuroscience Bulletin (2013)