Abstract
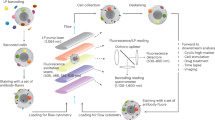
Flow cytometry allows high-content, multiparameter analysis of single cells, making it a promising tool for drug discovery and profiling of intracellular signaling. To add high-throughput capacity to flow cytometry, we developed a cell-based multiplexing technique called fluorescent cell barcoding (FCB). In FCB, each sample is labeled with a different signature, or barcode, of fluorescence intensity and emission wavelengths, and mixed with other samples before antibody staining and analysis by flow cytometry. Using three FCB fluorophores, we were able to barcode and combine entire 96-well plates, reducing antibody consumption 100-fold and acquisition time to 5–15 min per plate. Using FCB and phospho-specific flow cytometry, we screened a small-molecule library for inhibitors of T cell–receptor and cytokine signaling, simultaneously determining compound efficacy and selectivity. We also analyzed IFN-γ signaling in multiple cell types from primary mouse splenocytes, revealing differences in sensitivity and kinetics between B cells, CD4+ and CD4− T cells and CD11b-hi cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tung, J.W., Parks, D.R., Moore, W.A., Herzenberg, L.A. & Herzenberg, L.A. Identification of B-cell subsets: an exposition of 11-color (Hi-D) FACS methods. Methods Mol. Biol. 271, 37–58 (2004).
De Rosa, S.C., Herzenberg, L.A., Herzenberg, L.A. & Roederer, M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat. Med. 7, 245–248 (2001).
Krutzik, P.O., Irish, J.M., Nolan, G.P. & Perez, O.D. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: techniques and clinical applications. Clin. Immunol. 110, 206–221 (2004).
Krutzik, P.O. & Nolan, G.P. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A 55, 61–70 (2003).
Fleisher, T.A. et al. Detection of intracellular phosphorylated STAT-1 by flow cytometry. Clin. Immunol. 90, 425–430 (1999).
Sachs, K., Perez, O., Pe'er, D., Lauffenburger, D.A. & Nolan, G.P. Causal protein-signaling networks derived from multiparameter single-cell data. Science 308, 523–529 (2005).
Ilangumaran, S., Ramanathan, S., La Rose, J., Poussier, P. & Rottapel, R. Suppressor of cytokine signaling 1 regulates IL-15 receptor signaling in CD8+CD44high memory T lymphocytes. J. Immunol. 171, 2435–2445 (2003).
Krutzik, P.O., Clutter, M.R. & Nolan, G.P. Coordinate analysis of murine immune cell surface markers and intracellular phosphoproteins by flow cytometry. J. Immunol. 175, 2357–2365 (2005).
Ilangumaran, S., Finan, D. & Rottapel, R. Flow cytometric analysis of cytokine receptor signal transduction. J. Immunol. Methods 278, 221–234 (2003).
Kersh, E.N. et al. TCR signal transduction in antigen-specific memory CD8 T cells. J. Immunol. 170, 5455–5463 (2003).
Chow, S., Patel, H. & Hedley, D.W. Measurement of MAP kinase activation by flow cytometry using phospho-specific antibodies to MEK and ERK: potential for pharmacodynamic monitoring of signal transduction inhibitors. Cytometry 46, 72–78 (2001).
Zell, T. et al. Single-cell analysis of signal transduction in CD4 T cells stimulated by antigen in vivo. Proc. Natl. Acad. Sci. USA 98, 10805–10810 (2001).
Krutzik, P.O., Hale, M.B. & Nolan, G.P. Characterization of the murine immunological signaling network with phosphospecific flow cytometry. J. Immunol. 175, 2366–2373 (2005).
Irish, J.M. et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell 118, 217–228 (2004).
Clutter, M.R., Krutzik, P.O. & Nolan, G.P. Phospho-specific flow cytometry in drug discovery. Drug Discov. Today Technol. 2, 295–302 (2005).
Jacobberger, J.W. et al. Immunoreactivity of Stat5 phosphorylated on tyrosine as a cell-based measure of Bcr/Abl kinase activity. Cytometry A 54, 75–88 (2003).
Kuckuck, F.W., Edwards, B.S. & Sklar, L.A. High throughput flow cytometry. Cytometry 44, 83–90 (2001).
Edwards, B.S., Oprea, T., Prossnitz, E.R. & Sklar, L.A. Flow cytometry for high-throughput, high-content screening. Curr. Opin. Chem. Biol. 8, 392–398 (2004).
Nam, J.M., Thaxton, C.S. & Mirkin, C.A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 301, 1884–1886 (2003).
Fulton, R.J., McDade, R.L., Smith, P.L., Kienker, L.J. & Kettman, J.R., Jr. Advanced multiplexed analysis with the FlowMetrix system. Clin. Chem. 43, 1749–1756 (1997).
Vignali, D.A. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 243, 243–255 (2000).
Bradford, J.A., Buller, G., Suter, M., Ignatius, M. & Beechem, J.M. Fluorescence-intensity multiplexing: simultaneous seven-marker, two-color immunophenotyping using flow cytometry. Cytometry A 61, 142–152 (2004).
Zhang, J.H., Chung, T.D. & Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 (1999).
Nam, S. et al. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc. Natl. Acad. Sci. USA 102, 5998–6003 (2005).
Lagasse, E. & Weissman, I.L. Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods 197, 139–150 (1996).
Acknowledgements
We thank E. Danna and R. Wolkowicz for critical reading of the manuscript and J. Crane for technical support. P.O.K. was supported by a Howard Hughes Medical Institute predoctoral fellowship. G.P.N. was supported by National Heart Lung and Blood Institute contract N01-HV-28183 and National Institutes of Health grant AI35304.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.P.N. and P.O.K. are each paid consultants to Becton Dickenson, a provider of antibodies and flow cytometry–based reagents. G.P.N. consults occasionally with multiple pharmaceutical and biotechnology companies about flow cytometry–based analysis of signaling systems.
Supplementary information
Supplementary Fig. 1
Increasing cell number to antibody ratio does not significantly impact phospho-antibody staining. (PDF 486 kb)
Supplementary Fig. 2
Detailed titration of four hits from Jurkat drug screening experiment. (PDF 284 kb)
Rights and permissions
About this article
Cite this article
Krutzik, P., Nolan, G. Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat Methods 3, 361–368 (2006). https://doi.org/10.1038/nmeth872
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth872
This article is cited by
-
High-dimensional multi-pass flow cytometry via spectrally encoded cellular barcoding
Nature Biomedical Engineering (2023)
-
Different NIPBL requirements of cohesin-STAG1 and cohesin-STAG2
Nature Communications (2023)
-
Screening hydrogels for antifibrotic properties by implanting cellularly barcoded alginates in mice and a non-human primate
Nature Biomedical Engineering (2023)
-
Impaired JAK-STAT pathway signaling in leukocytes of the frail elderly
Immunity & Ageing (2022)
-
The metabolic profile of reconstituting T-cells, NK-cells, and monocytes following autologous stem cell transplantation and its impact on outcome
Scientific Reports (2022)