Abstract
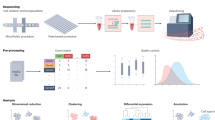
Cell and protein arrays have demonstrated remarkable utility in the high-throughput evaluation of biological responses; however, they lack the complexity of native tissue and organs. Here we spotted tissue extracellular matrix (ECM) particles as two-dimensional (2D) arrays or incorporated them with cells to generate three-dimensional (3D) cell-matrix microtissue arrays. We then investigated the responses of human stem, cancer and immune cells to tissue ECM arrays originating from 11 different tissues. We validated the 2D and 3D arrays as representative of the in vivo microenvironment by means of quantitative analysis of tissue-specific cellular responses, including matrix production, adhesion and proliferation, and morphological changes after culture. The biological outputs correlated with tissue proteomics, and network analysis identified several proteins linked to cell function. Our methodology enables broad screening of ECMs to connect tissue-specific composition with biological activity, providing a new resource for biomaterials research and further understanding of regeneration and disease mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Hay, E.D. Extracellular matrix. J. Cell Biol. 91, 205s–223s (1981).
Watt, F.M. & Huck, W.T.S. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 14, 467–473 (2013).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Lee, G.Y., Kenny, P.A., Lee, E.H. & Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 4, 359–365 (2007).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
Kim, M.S. et al. A draft map of the human proteome. Nature 509, 575–581 (2014).
Uhlén, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Anderson, D.G., Levenberg, S. & Langer, R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 22, 863–866 (2004).
Mei, Y. et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 9, 768–778 (2010).
Flaim, C.J., Chien, S. & Bhatia, S.N. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods 2, 119–125 (2005).
Reticker-Flynn, N.E. et al. A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis. Nat. Commun. 3, 1122 (2012).
Gobaa, S. et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods 8, 949–955 (2011).
Dolatshahi-Pirouz, A. et al. A combinatorial cell-laden gel microarray for inducing osteogenic differentiation of human mesenchymal stem cells. Sci. Rep. 4, 3896 (2014).
Woodruff, K., Fidalgo, L.M., Gobaa, S., Lutolf, M.P. & Maerkl, S.J. Live mammalian cell arrays. Nat. Methods 10, 550–552 (2013).
Ranga, A. et al. 3D niche microarrays for systems-level analyses of cell fate. Nat. Commun. 5, 4324 (2014).
Mouw, J.K., Ou, G. & Weaver, V.M. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 15, 771–785 (2014).
Atala, A., Bauer, S.B., Soker, S., Yoo, J.J. & Retik, A.B. Tissue-engineered autologous bladders for patients needing cytoplasty. Lancet 367, 1241–1246 (2006).
Macchiarini, P. et al. Clinical transplantation of a tissue-engineered airway. Lancet 372, 2023–2030 (2008).
Sicari, B.M. et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 6, 234ra258 (2014).
Ott, H.C. et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 (2008).
Uygun, B.E. et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 16, 814–820 (2010).
Petersen, T.H. et al. Tissue-engineered lungs for in vivo implantation. Science 329, 538–541 (2010).
Song, J.J. et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 19, 646–651 (2013).
Wu, I., Nahas, Z., Kimmerling, K.A., Rosson, G.D. & Elisseeff, J.H. An injectable adipose matrix for soft-tissue reconstruction. Plast. Reconstr. Surg. 129, 1247–1257 (2012).
Kononen, J. et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med. 4, 844–847 (1998).
Engler, A.J., Sen, S., Sweeney, H.L. & Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Bissell, M.J. & Hines, W.C. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 17, 320–329 (2011).
Badylak, S.F., Valentin, J.E., Ravindra, A.K., McCabe, G.P. & Stewart-Akers, A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng. Part A 14, 1835–1842 (2008).
DeForest, C.A., Polizzotti, B.D. & Anseth, K.S. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater. 8, 659–664 (2009).
Tibbitt, M.W. & Anseth, K.S. Dynamic microenvironments: the fourth dimension. Sci. Transl. Med. 4, 160ps124 (2012).
Badylak, S.F., Lantz, G.C., Coffey, A. & Geddes, L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 47, 74–80 (1989).
Nerem, R.M. Regenerative medicine: the emergence of an industry. J. R. Soc. Interface 7, S771–S775 (2010).
Crapo, P.M., Gilbert, T.W. & Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Kuribayashi, H. et al. Clinicopathological analysis of primary lung carcinoma with heterotopic ossification. Lung Cancer 64, 160–165 (2009).
Averill, M.M., Kerkhoff, C. & Bornfeldt, K.E. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler. Thromb. Vasc. Biol. 32, 223–229 (2012).
Vilen, S.T. et al. Trypsin-2 enhances carcinoma invasion by processing tight junctions and activating ProMT1-MMP. Cancer Invest. 30, 583–592 (2012).
Vizcaíno, J.A. et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 32, 223–226 (2014).
Naba, A. et al. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteomics 11, M111.014647 (2012).
Yan, P., Seelentag, W., Bachmann, A. & Bosman, F.T. An agarose matrix facilitates sectioning of tissue microarray blocks. J. Histochem. Cytochem. 55, 21–24 (2007).
Yoshimura, K. et al. Integrin α2 mediates selective metastasis to the liver. Cancer Res. 69, 7320–7328 (2009).
Mitchell, J.B. et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24, 376–385 (2006).
Bratt-Leal, A.M., Carpenedo, R.L., Ungrin, M.D., Zandstra, P.W. & McDevitt, T.C. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials 32, 48–56 (2011).
Acknowledgements
The authors acknowledge funding through a Maryland Stem Cell Investigator-Initiated Grant, Department of Defense Grant W81XWH-l1-2-0022, the Armed Forces Institute of Regenerative Medicine, and the Jules Stein Chair from Research to Prevent Blindness. The authors thank M. Frisk for carefully editing the manuscript and providing insightful suggestions. V.Z.B. and M.T.W. thank the Hartwell Foundation for postdoctoral fellowships. A.P. is supported by National Cancer Institute's Clinical Proteomic Tumor Analysis Consortium initiative (U24CA160036). J.H.E. acknowledges a Johns Hopkins Tilghman Fellowship Sabbatical Grant and a Swiss National Foundation Visiting Professor Grant along with M. Swartz and the Swartz lab for hosting a semester sabbatical. We acknowledge A. Ewald (Departments of Cell Biology and Oncology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA) for discussions on the systems biology analysis and for providing mammary tumors for processing and array spotting. We thank R. Cole and L. DeVine of the Johns Hopkins School of Medicine Mass Spectrometry and Proteomics Facility for mass spectrometry preparation and analysis.
Author information
Authors and Affiliations
Contributions
V.Z.B. and J.H.E. conceived the idea. V.Z.B. made 2D and 3D tissue arrays. V.Z.B. performed stem cell studies. M.R.B. and H.J. developed seeding chambers and performed cancer adhesion and proliferation studies. K.S. and M.T.W. performed macrophage studies. K.S. performed gel electrophoresis work and collagen ECM classification. S.S.M., V.Z.B., M.T.W. and A.P. performed proteomic analysis. D.P. provided melanoma lines and guidance for cancer studies. M.T.W. and J.S.B. performed systems analysis. V.Z.B., M.T.W. and J.H.E. evaluated all data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.E. has a financial interest in a specific tissue ECM technology, acellular adipose tissue.
Integrated supplementary information
Supplementary Figure 1 Characterization of tissue ECM spots.
(a) SDS-PAGE gel of intact tissue particles and pepsin digested particles (representative of n = 2 technical replicates). Qualitative protein composition of different tissue ECM types before and after pepsin processing is visualized as dark bands and regions with decreasing protein size from top to bottom. High molecular weight bands visible in intact tissue particles are not present after pepsin digestion (arrows). (b) Topographical features of array spots were examined using scanning electron microscopy and showed structural differences between tissue types (representative of 3 images from 1 array spot). Scale bar, 10 μm. (c) Nanoindentation testing was performed to measure the surface mechanical properties of the dried particle spots on tissue arrays. The elastic modulus and hardness are shown for 6 different tissue sources (8-10 technical replicate measurements were conducted per tissue, and measurements defined as outliers by Grubbs’ test were excluded; n = 9 bladder, n = 8 brain, n = 9 cardiac, n = 9 liver, n = 7 lung, n = 7 spleen). Data are mean ± s.d. Mechanical properties were analyzed by one-way ANOVA with a post-hoc Tukey test. Statistically significant increases (P < 0.05) are signified with (*) and the abbreviations of the tissues they are greater than: Bl=bladder, Br-brain, Cdc=cardiac, Lv=liver, Lg=lung, Sp=spleen.
Supplementary Figure 2 Tissue array cell-seeding method optimization.
(a) Tissue microarrays were designed to randomize the location of each type of tissue spot to limit error associated with cell seeding. When the two spot patterns shown are used in duplicate (n = 6 array spots per tissue type), each spot type is distributed between the inner and outer columns and inlet and outlet rows. (b) A polydimethylsiloxane (PDMS) compartment was designed to confine medium flow and promote uniform cell seeding. (c) Variability associated with cell seeding in PDMS compartments on control microarray chips (all spots soluble collagen control) was less than 15% between rows and columns (coefficient of variation, n = 10 rows, n = 4 columns, n = 2 array chips). Data are mean.
Supplementary Figure 3 Optimization of cell-tissue ECM for formation of breast cancer–tissue ECM 3D spheroids.
Cells were suspended at 500,000 cells/mL and added to tissue suspended at 0.25–4 mg/ml at a 1:1 v/v ratio in a 40 μL hanging drop. (a) Tissue particles at high concentrations could disrupt spheroid formation, but all tissues screened were compatible with formation of compact cell-tissue spheroids at tissue particle concentrations of 1 mg/ml or less after 6 days of culture (representative images of n = 3 array spots for 1 mg/ml & 0.5 mg/ml or n = 2 for 4 mg/ml, 2 mg/ml & 0.25 mg/ml). (b) Spheroid formation began with the development of several small spheroids that coalesced to form a single compact spheroid after 6 days with a tissue concentration of 1 mg/ml (representative images of n = 3 array spots). (c) The viability/proliferation of cells after 6 days in culture increased with increasing tissue concentration up to 1 mg/ml (n = 3 array spots for 1 mg/ml & 0.5 mg/ml or n = 2 for 4 mg/ml, 2 mg/ml & 0.25 mg/ml). At concentrations of 2-4 mg/ml cell viability/proliferation decreased for several tissue types. Data are mean ± s.d. Scale bars = 1 mm.
Supplementary Figure 4 Array spots generated from diseased tissues.
Diseased tissue arrays were fabricated by processing tissue taken from murine mammary tumors and skeletal muscle of mutant dystrophic mice. Tissue was processed similar to normal tissue and spotted in a microarray with normal tissue controls. Histological staining patterns with hematoxylin and eosin (H&E) and Masson’s trichrome are different for normal mammary, early stage tumor, and late stage tumor tissue. Increased blue staining with Masson’s trichrome indicates higher levels of collagen content (n = 3 array spots). Scale bar = 20 μm.
Supplementary Figure 5 Fidelity of tissue processing, array spotting and proteomic analysis.
(a) Images of Masson’s Trichrome stained tissue spots (representative of n = 3 array spots) were compared to determine the uniformity of tissue array spots and the variability associated with array fabrication. (b) Staining characteristics were quantified by calculating different hue levels in ImageJ, and demonstrated that variability remained below 25% for the predominant color of all tissues except brain, kidney, and small intestine (coefficient of variation, n = 3 array spots). Data are mean ± s.d. (c) Proteomic comparison of n = 3 different spot suspensions of processed small intestine tissue confirmed consistency in the abundance (PSMs) and number of total unique ECM and non-ECM proteins identified. (d) Proteomics analysis confirmed most proteins were identified in at least two of three different small intestine spot suspensions with greater consistency for higher abundance proteins (greater than 1 PSM) than rare proteins (1 PSM).
Supplementary Figure 6 Characterization and protein composition of tissue arrays prepared with multiple processing methods.
Porcine lung and cardiac tissues were treated using various degrees of chemical processing; mild (10 min peracetic acid & 18 hr DNAse), moderate (3 hr peracetic acid, 18 hr Triton X-100 & 18 hr DNAse), harsh (moderate treatment + 16 hr SDS), and digest (moderate treatment + pepsin digestion). (a) The protein composition of tissue ECM prepared with different methods of processing show distinct banding patterns on an SDS-PAGE gel (representative of n = 2 technical replicates). (b) Decellularized particles of each type were spotted onto arrays. Cardiac and lung tissue array spots were stained with Masson’s trichrome to show compositional variability following different degrees of processing. An example of combining particulate mixtures of moderately processed lung and cardiac ECM at different ratios (wt/wt) is also provided (representative images of n = 3 array spots for each condition).
Supplementary Figure 7 Comparison of macrophage morphology on arrays prepared with different processing methods.
(a) Macrophage morphology on each tissue ECM formulation was segmented using an automated algorithm (cell perimeter/segmentation outlined in blue). (b) Cell spreading on mixtures of moderately processed lung:cardiac tissue and (c) on single tissue spots with different degrees of processing (representative images of n = 3 array spots). (d) Quantification of cell morphology by the convex hull perimeter to area ratio from segmented images of cells cultured on fresh, digested, and treated cardiac and lung tissue. Data are mean ± s.d. Scale bars = 200 μm. Morphology for each tissue type was analyzed by a student’s t-test with the Bonferroni correction. Statistically significant increases (P < 0.05) between different processing methods within a tissue are signified with (*) and the label/abbreviations of the tissues they are greater than: fresh, mild, mod=moderate, harsh, or digest. Ratios of lung to cardiac tissue particles are signified as 1:3, 3:1, and 1:1.
Supplementary Figure 8 Cellular differentiation and viability in 3D spheroids.
(a) Osteogenic gene expression of hASCs cultured in 3D bone and lung tissue ECM microtissues. hASC microtissue spheroids were formed in growth media (GM) for 7 days, and either kept in GM or switched to osteogenic media (OM) for an additional 14 days. Expression of the late stage osteogenesis gene osteocalcin was determined via qRT-PCR relative to the day 7 time point (3 technical replicates of n = 3 pooled spheroids) in both GM (top) and OM (bottom) media conditions. Data are means ± s.d. Differences in osteocalcin expression between 7 and 21 days for each condition were determined by a Student’s t-test (*P < 0.05). (b) Breast cancer cells labeled with Cell Tracker or calcein AM were fluorescently imaged on 2D tissue arrays over 7 days to quantify cell number. Cardiac tissue supported the greatest number of cells after 7 days, while bone and kidney tissues had the fewest (n = 9 array spots). Data not available for day 7 soluble collagen control (cells formed a monolayer and peeled from spot during culture) or day 3 liver (autofluorescence tissue too high for Cell Tracker imaging). (c) Cell viability and proliferation as assessed by Alamar Blue assay for 3D tissue spheroids decreased for all tissue types over 7 days but was greatest for cartilage tissue containing spheroids (n = 3 spheroids). Data are mean ± s.d.
Supplementary Figure 9 Attachment of bone marrow–derived macrophages to different tissue substrates on array.
Cells were polarized toward an M1 or M2 phenotype with cytokine stimulation, allowed to attach for 24 h, and fixed. Actin cytoskeleton is stained with phalloidin (representative image of n = 3 array spots). Scale bars, 200 μm.
Supplementary Figure 10 In vitro and in vivo macrophage polarization in response to tissue ECM.
(a) Macrophage phenotype on bone and collagen array spots after 24 hours in polarizing conditions was determined by immunofluorescence staining for Arginase-1 (green) and iNOS (red) (representative of n=3 array spots, scale bar=100 μm). (b) Bone tissue ECM and collagen were hydrated and injected subcutaneously into wild type C57BL/6 mice. Implants were harvested after one week for immunofluorescence staining for iNOS (green) and F4/80 (red) (representative of 4 images from n=2 animals). Scale bars=100 μm. Heat map representation of the effect of 2D tissue ECM substrates on macrophage gene expression. Primary mouse bone marrow derived macrophages were cultured on 2D bone, cardiac, liver, lung, or spleen ECM and compared to uncoated polystyrene in M0 (non-polarized), M1 (LPS + IFNγ), and M2 (IL4) cytokine environments for 24 hours. Relative gene expression of IL1β, iNOS TNFα, Arg1, Fizz1, and IL10 was determined via qRT-PCR, and is displayed as ΔΔCt over the tissue culture plastic (TCP) control in each media condition (triplicate wells of n = 3 macrophage isolations). Hierarchical clustering analysis was conducted for each cytokine environment. Macrophage gene expression was analyzed by one-way ANOVA with a post-hoc Tukey test, and these statistical results are provided in Supplementary Table 1.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1878 kb)
Supplementary Table 1
One-way ANOVA results for cancer cells and macrophages on 2D tissue arrays. (XLSX 18 kb)
Supplementary Table 2
Results of correlation analysis between ECM protein quantity and in vitro assay outcomes. (XLSX 2271 kb)
Supplementary Table 3
Osteogenesis gene ontology enrichment analysis results for tissue ECMs. (XLSX 11 kb)
Rights and permissions
About this article
Cite this article
Beachley, V., Wolf, M., Sadtler, K. et al. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat Methods 12, 1197–1204 (2015). https://doi.org/10.1038/nmeth.3619
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3619
This article is cited by
-
Anti-Osteoarthritic Effects of Cartilage-Derived Extracellular Matrix in a Rat Osteoarthritis Model
Tissue Engineering and Regenerative Medicine (2023)
-
An immunologically active, adipose-derived extracellular matrix biomaterial for soft tissue reconstruction: concept to clinical trial
npj Regenerative Medicine (2022)
-
Emerging Biomimetic Materials for Studying Tumor and Immune Cell Behavior
Annals of Biomedical Engineering (2020)
-
The two faces of titanium dioxide nanoparticles bio-camouflage in 3D bone spheroids
Scientific Reports (2019)
-
Host tissue determinants of tumour immunity
Nature Reviews Cancer (2019)