Abstract
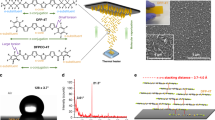
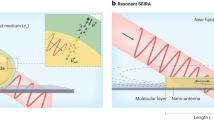
π-Conjugated organic semiconductors have been explored in several optoelectronic devices, yet their use in molecular detection as surface-enhanced Raman spectroscopy (SERS)-active platforms is unknown. Herein, we demonstrate that SERS-active, superhydrophobic and ivy-like nanostructured films of a molecular semiconductor, α,ω-diperfluorohexylquaterthiophene (DFH-4T), can be easily fabricated by vapour deposition. DFH-4T films without any additional plasmonic layer exhibit unprecedented Raman signal enhancements up to 3.4 × 103 for the probe molecule methylene blue. The combination of quantum mechanical computations, comparative experiments with a fluorocarbon-free α,ω-dihexylquaterthiophene (DH-4T), and thin-film microstructural analysis demonstrates the fundamental roles of the π-conjugated core fluorocarbon substitution and the unique DFH-4T film morphology governing the SERS response. Furthermore, Raman signal enhancements up to ∼1010 and sub-zeptomole (<10−21 mole) analyte detection were accomplished by coating the DFH-4T films with a thin gold layer. Our results offer important guidance for the molecular design of SERS-active organic semiconductors and easily fabricable SERS platforms for ultrasensitive trace analysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hudson, S. D. & Chumanov, G. Bioanalytical applications of SERS (surface-enhanced Raman spectroscopy). Anal. Bioanal. Chem. 394, 679–686 (2009).
Pearman, W. F. & Fountain, A. W. Classification of chemical and biological warfare agent simulants by surface-enhanced Raman spectroscopy and multivariate statistical techniques. Appl. Spectrosc. 60, 356–365 (2006).
Halvorson, R. A. & Vikesland, P. J. Surface-enhanced Raman spectroscopy (SERS) for environmental analyses. Environ. Sci. Technol. 44, 7749–7755 (2010).
Wang, L. et al. Simple, rapid, sensitive, and versatile SWNT-paper sensor for environmental toxin detection competitive with ELISA. Nano Lett. 9, 4147–4152 (2009).
Huang, N., Lü, T., Zhang, R. & Cao, W. High sensitivity gravimetric sensor made of carbon fiber epoxy composite on Pb (Mg1/3Nb2/3) O3-PbTiO3 single crystal substrate. Appl. Phys. Lett. 103, 053507 (2013).
Guo, X., Ying, Y. & Tong, L. Photonic nanowires: From subwavelength waveguides to optical sensors. Acc. Chem. Res. 47, 656–666 (2013).
Chen, A. & Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 42, 5425–5438 (2013).
Schlücker, S. Surface-enhanced Raman spectroscopy: concepts and chemical applications. Angew. Chem. Int. Ed. 53, 4756–4795 (2014).
Lombardi, J. R. & Birke, R. L. A unified view of surface-enhanced Raman scattering. Acc. Chem. Res. 42, 734–742 (2009).
Alessandri, I. Enhancing Raman scattering without plasmons: unprecedented sensitivity achieved by TiO2 shell-based resonators. J. Am. Chem. Soc. 135, 5541–5544 (2013).
Wang, Y. et al. Direct observation of surface-enhanced Raman scattering in ZnO nanocrystals. J. Raman Spectrosc. 40, 1072–1077 (2009).
Li, W. et al. CuTe nanocrystals: shape and size control, plasmonic properties, and use as SERS probes and photothermal agents. J. Am. Chem. Soc. 135, 7098–7101 (2013).
Quagliano, L. G. Observation of molecules adsorbed on III–V semiconductor quantum dots by surface-enhanced Raman scattering. J. Am. Chem. Soc. 126, 7393–7398 (2004).
Chen, L. Y., Yu, J. S., Fujita, T. & Chen, M. W. Nanoporous copper with tunable nanoporosity for SERS applications. Adv. Funct. Mater. 19, 1221–1226 (2009).
Facchetti, A. π-conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 23, 733–758 (2010).
Labastide, J. et al. Directional charge separation in isolated organic semiconductor crystalline nanowires. Nat. Commun. 7, 10629 (2016).
Zhang, L. et al. Unconventional, chemically stable, and soluble two-dimensional angular polycyclic aromatic hydrocarbons: from molecular design to device applications. Acc. Chem. Res. 48, 500–509 (2015).
Zang, L., Che, Y. & Moore, J. S. One-dimensional self-assembly of planar π-conjugated molecules: adaptable building blocks for organic nanodevices. Acc. Chem. Res. 41, 1596–1608 (2008).
Capelli, R. et al. Organic light-emitting transistors with an efficiency that outperforms the equivalent light-emitting diodes. Nat. Mater. 9, 496–503 (2010).
Usta, H., Facchetti, A. & Marks, T. J. n-Channel semiconductor materials design for organic complementary circuits. Acc. Chem. Res. 44, 501–510 (2011).
Samuel, I. D. W. & Turnbull, G. A. Organic semiconductor lasers. Chem. Rev. 107, 1272–1295 (2007).
Roberts, M. E. et al. Water-stable organic transistors and their application in chemical and biological sensors. Proc. Natl Acad. Sci. USA 105, 12134–12139 (2008).
Kuribara, K. et al. Organic transistors with high thermal stability for medical applications. Nat. Commun. 3, 723 (2012).
Yilmaz, M. et al. Micro-/nanostructured highly crystalline organic semiconductor films for surface-enhanced Raman spectroscopy applications. Adv. Funct. Mater. 25, 5669–5676 (2015).
Yilmaz, M. et al. Combining 3-D plasmonic gold nanorod arrays with colloidal nanoparticles as a versatile concept for reliable, sensitive, and selective molecular detection by SERS. Phys. Chem. Chem. Phys. 16, 5563–5570 (2014).
Demirel, G., Malvadkar, N. & Demirel, M. C. Template-based and template-free preparation of nanostructured parylene via oblique angle polymerization. Thin Solid Films 518, 4252–4255 (2010).
Facchetti, A. et al. Building blocks for n-type molecular and polymeric electronics. Perfluoroalkyl-versus alkyl-functionalized oligothiophenes (nTs; n = 2–6). Systematic synthesis, spectroscopy, electrochemistry, and solid-state organization. J. Am. Chem. Soc. 126, 13480–13501 (2004).
Zhang, Y. et al. Intrinsic and extrinsic parameters for controlling the growth of organic single-crystalline nanopillars in photovoltaics. Nano Lett. 14, 5547–5554 (2014).
Akin, M. S. et al. Large area uniform deposition of silver nanoparticles through bio-inspired polydopamine coating on silicon nanowire arrays for practical SERS applications. J. Mater. Chem. B 2, 4894–4900 (2014).
Xu, W. et al. Surface enhanced Raman spectroscopy on a flat graphene surface. Proc. Natl Acad. Sci. USA 109, 9281–9286 (2012).
Sharma, B., Frontiera, R. R., Henry, A.-I., Ringe, E. & Van Duyne, R. P. SERS: materials, applications, and the future. Mater. Today 15, 16–25 (January, 2012).
Dinelli, F. et al. High-mobility ambipolar transport in organic light-emitting transistors. Adv. Mater. 18, 1416–1420 (2006).
Gieseking, R. L., Ratner, M. A. & Schatz, G. C. Theoretical modeling of voltage effects and the chemical mechanism in surface-enhanced Raman scattering. Faraday Discuss. http://dx.doi.org/10.1039/C7FD00122C (2017).
Jensen, L., Zhao, L. L., Autschbach, J. & Schatz, G. C. Theory and method for calculating resonance Raman scattering from resonance polarizability derivatives. J. Chem. Phys. 123, 174110 (2005).
Jensen, L., Autschbach, J. & Schatz, G. C. Finite lifetime effects on the polarizability within time-dependent density-functional theory. J. Chem. Phys. 122, 224115 (2005).
Tokura, Y. & Koda, T. Experimental determination of the charge-transfer exciton band width in anthracene-PMDA crystal. Solid State Commun. 40, 299–301 (1981).
Facchetti, A. et al. Building blocks for n-type molecular and polymeric electronics. Perfluoroalkyl- versus alkyl-functionalized oligothiophenes (nT; n = 2–6). Part 2. Thin film microstructure, semiconductor performance, and modeling to charge injection in field-effect transistors. J. Am. Chem. Soc. 126, 13859–13874 (2004).
Zou, S. & Schatz, G. C. Silver nanoparticle array structures that produce giant enhancements in electromagnetic fields. Chem. Phys. Lett. 403, 62–67 (2005).
Fan, M. & Brolo, A. G. Silver nanoparticles self assembly as SERS substrates with near single molecule detection limit. Phys. Chem. Chem. Phys. 11, 7381–7389 (2009).
Pandey, P. A. et al. Physical vapor deposition of metal nanoparticles on chemically modified graphene: observations on metal-graphene interactions. Small 7, 3202–3210 (2011).
Ding, S.-Y. et al. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 1, 16021 (2016).
Wei, W., Chen, K. & Ge, G. Strongly coupled nanorod vertical arrays for plasmonic sensing. Adv. Mater. 25, 3863–3868 (2013).
Doherty, M. D., Murphy, A., McPhillips, J., Pollard, R. J. & Dawson, P. Wavelength dependence of Raman enhancement of gold nanorod arrays: quantitative experiment and modelling of a hot spot dominated system. J. Phys. Chem. C 114, 19913–19919 (2010).
Ghenuche, P., Cherukulappurath, S., Taminiau, T. H., van Hulst, N. F. & Quidant, R. Spectroscopic mode mapping of resonant plasmon nanoantennas. Phys. Rev. Lett. 101, 116805 (2008).
Boerigter, C., Campana, R., Morabito, M. & Linic, S. Evidence and implications of direct charge excitation as the dominant mechanism in plasmon-mediated photocatalysis. Nat. Commun. 7, 10545 (2016).
Wang, X., Shi, W., She, G. & Mu, L. Using Si and Ge nanostructures as substrates for surface-enhanced Raman scattering based on photoinduced charge transfer mechanism. J. Am. Chem. Soc. 133, 16518–16523 (2011).
Lombardi, J. R. & Birke, R. L. Theory of surface-enhanced Raman scattering in semiconductors. J. Phys. Chem. C 118, 11120–11130 (2014).
Chai, J.-D. & Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008).
Chai, J.-D. & Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 128, 84106 (2008).
Dunning, T. H. Jr Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007–1023 (1989).
Körzdörfer, T., Parrish, R. M., Sears, J. S., Sherrill, C. D. & Brédas, J. L. On the relationship between bond-length alternation and many-electron self-interaction error. J. Chem. Phys. 137, 124305 (2012).
Shao, Y. et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 113, 184–215 (2015).
Johnson, R. D. III NIST computational chemistry comparison and benchmark database. NIST Standard Reference Database Number 101 (2013); http://cccbdb.nist.gov
Ridley, J. & Zerner, M. An intermediate neglect of differential overlap technique for spectroscopy: Pyrrole and the azines. Theor. Chim. Acta 32, 111–134 (1973).
Fox, T., Kotzian, M. & Rosch, N. Design of rigid donor–acceptor systems with a low-lying charge-transfer state. An INDO model study of barrelene-based compounds. J. Phys. Chem. 97, 11420–11426 (1993).
Nazeeruddin, M. K. et al. DFT-INDO/S modeling of new high molar extinction coefficient charge-transfer sensitizers for solar cell applications. Inorg. Chem. 45, 787–797 (2006).
Stewart, J. J. P. MOPAC: a semiempirical molecular orbital program. J. Comput. Aided Mol. Des. 4, 1–105 (1990).
Shapley, W. A., Reimers, J. R. & Hush, N. S. INDO/S parameters for gold. Int. J. Quantum Chem. 90, 424–438 (2002).
Acknowledgements
This work was partially supported by Gazi University (grant no. 05/2015-19) and Polyera Corporation. G.D., H.U. and Y.D. acknowledge support from the Turkish Academy of Sciences, Distinguished Young Scientist Award (TUBA-GEBIP). A.F. thanks the Shenzhen Peacock Plan project (KQTD20140630110339343) and the BSF (AGMT-2012250///02).
Author information
Authors and Affiliations
Contributions
G.D., H.U. and A.F. conceived and designed the experiments. H.U., M.Ö. and A.F. synthesized the small molecular organic semiconductors. G.D., M.Y., U.T. and E.B. fabricated the nanostructured platforms and performed the experiments. G.C.S. and R.L.G. designed and performed the theoretical calculations. Y.D. helped with the density functional theory calculations. All authors discussed the results and co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2271 kb)
Rights and permissions
About this article
Cite this article
Yilmaz, M., Babur, E., Ozdemir, M. et al. Nanostructured organic semiconductor films for molecular detection with surface-enhanced Raman spectroscopy. Nature Mater 16, 918–924 (2017). https://doi.org/10.1038/nmat4957
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4957
This article is cited by
-
Recent development of surface-enhanced Raman scattering for biosensing
Journal of Nanobiotechnology (2023)
-
Ultrafast charge transfer in mixed-dimensional WO3-x nanowire/WSe2 heterostructures for attomolar-level molecular sensing
Nature Communications (2023)
-
Surfactant-free interfacial growth of graphdiyne hollow microspheres and the mechanistic origin of their SERS activity
Nature Communications (2023)
-
The Fabrication and SERS Performance of Multi-layer Hollow Au-Ag Alloy Nano Urchins Structure-based SERS Fiber Probe
Journal of Wuhan University of Technology-Mater. Sci. Ed. (2023)
-
Nanomaterials meet surface-enhanced Raman scattering towards enhanced clinical diagnosis: a review
Journal of Nanobiotechnology (2022)