Abstract
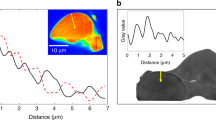
Biomineralization integrates complex processes leading to an extraordinary diversity of calcareous biomineral crystalline architectures, in intriguing contrast with the consistent presence of a sub-micrometric granular structure. Hence, gaining access to the crystalline architecture at the mesoscale, that is, over a few granules, is key to building realistic biomineralization scenarios. Here we provide the nanoscale spatial arrangement of the crystalline structure within the ‘single-crystalline’ prisms of the prismatic layer of a Pinctada margaritifera shell, exploiting three-dimensional X-ray Bragg ptychography microscopy. We reveal the details of the mesocrystalline organization, evidencing a crystalline coherence extending over a few granules. We additionally prove the existence of larger iso-oriented crystalline domains, slightly misoriented with respect to each other, around one unique rotation axis, and whose shapes are correlated with iso-strain domains. The highlighted mesocrystalline properties support recent biomineralization models involving partial fusion of oriented nanoparticle assembly and/or liquid droplet precursors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Weiner, S. & Addadi, L. Design strategies in mineralized biological materials. J. Mater. Chem. 7, 689–702 (1997).
Boggild, O. M. The shell structure of molluscs. Kgl Dan. Vidensk Selsk Skr. Natruvidensk Og Mathem 2, 231–236 (1930).
Grégoire, C. in Mollusca Vol. VII (eds Florkin, M. & Scheer, B. T.) 45–145 (Academic, 1972).
Taylor, J. D. & Kennedy, W. J. The shell structure and mineralogy of the Bivalvia: introduction, Nuculacea trigonacea. Bull. Br. Mus. Nat. Hist. 3, 1–125 (1969).
Cuif, J.-P. et al. Evidence of a biological control over origin, growth and end of the calcite prisms in the shells of Pinctada margaritifera (Pelecypod, Pterioidea). Minerals 4, 815–834 (2014).
Farre, B. et al. Shell layers of the black-lip pearl oyster Pinctada margaritifera: matching microstructure and composition. Comp. Biochem. Physiol. B 159, 131–139 (2011).
Dauphin, Y. et al. Structure and composition of the nacre–prisms transition in the shell of Pinctada margaritifera (Mollusca, Bivalvia). Anal. Bioanal. Chem. 390, 1659–1669 (2008).
Dauphin, Y. The nanostructural unity of Mollusc shells. Mineral. Mag. 72, 243–246 (2008).
Sethmann, I., Hinrichs, R., Wörheide, G. & Putnis, A. Nano-cluster composite structure of calcitic sponge spicules—a case study of basic characteristics of biominerals. J. Inorg. Biochem. 100, 88–96 (2006).
Baronnet, A., Cuif, J. P., Dauphin, Y., Farre, B. & Nouet, J. Crystallization of biogenic Ca-carbonate within organo-mineral micro-domains. Structure of the calcite prisms of the Pelecypod Pinctada margaritifera (Mollusca) at the submicron to nanometre ranges. Mineral. Mag. 72, 617–626 (2008).
Dauphin, Y. Soluble organic matrices of the calcitic prismatic shell layers of two Pteriomorphid Bivalves Pinna nobilis and Pinctada margaritifera. J. Biol. Chem. 278, 15168–15177 (2003).
Jacob, D. E. et al. Nanostructure, composition and mechanisms of bivalve shell growth. Geochim. Cosmochim. Acta 72, 5401–5415 (2008).
Cölfen, H. & Antonietti, M. Mesocrystals: inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. 44, 5576–5591 (2005).
Berman, A. et al. Biological control of crystal texture: a widespread strategy for adapting crystal properties to function. Science 259, 776–779 (1993).
Gilow, C., Zolotoyabko, E., Paris, O., Fratzl, P. & Aichmayer, B. Nanostructure of biogenic calcite crystals: a view by small-angle X-ray scattering. Cryst. Growth Des. 11, 2054–2058 (2011).
Pokroy, B. et al. Anisotropic lattice distortions in biogenic calcite induced by intra-crystalline organic molecules. J. Struct. Biol. 155, 96–103 (2006).
Pokroy, B., Fitch, A. & Zolotoyabko, E. The microstructure of biogenic calcite: a view by high-resolution synchrotron powder diffraction. Adv. Mater. 18, 2363–2368 (2006).
Okumura, T., Suzuki, M., Nagasawa, H. & Kogure, T. Microstructural variation of biogenic calcite with intracrystalline organic macromolecules. Cryst. Growth Des. 12, 224–230 (2012).
Okumura, T., Suzuki, M., Nagasawa, H. & Kogure, T. Characteristics of biogenic calcite in the prismatic layer of a pearl oyster, Pinctada fucata. Micron 41, 821–826 (2010).
Gilbert, P. U. P. A., Young, A. & Coppersmith, S. N. Measurement of c-axis angular orientation in calcite (CaCO3) nanocrystals using X-ray absorption spectroscopy. Proc. Natl Acad. Sci. USA 108, 11350–11355 (2011).
Olson, I. C. et al. Crystal lattice tilting in prismatic calcite. J. Struct. Biol. 183, 180–190 (2013).
Berman, A. et al. Intercalation of sea urchin proteins in calcite: study of a crystalline composite material. Science 250, 664–667 (1990).
Sayre, D. Some implications of a theorem due to Shannon. Acta Crystallogr. 5, 843 (1952).
Miao, J., Charalambous, P., Kirz, J. & Sayre, D. Extending the methodology of X-ray crystallography to allow imaging of micrometre-sized non-crystalline specimens. Nature 400, 342–344 (1999).
Miao, J., Ishikawa, T., Robinson, I. K. & Murnane, M. M. Beyond crystallography: diffractive imaging using coherent X-ray light sources. Science 348, 530–535 (2015).
Ayyer, K. et al. Macromolecular diffractive imaging using imperfect crystals. Nature 530, 202–206 (2016).
Büttner, F. et al. Dynamics and inertia of skyrmionic spin structures. Nat. Phys. 11, 225–228 (2015).
Ulvestad, A. et al. Topological defect dynamics in operando battery nanoparticles. Science 348, 1344–1347 (2015).
Chamard, V. et al. Three-dimensional X-ray Fourier transform holography: the Bragg case. Phys. Rev. Lett. 104, 165501 (2010).
Rodenburg, J. M. & Bates, R. H. T. The theory of super-resolution electron microscopy via Wigner-distribution deconvolution. Phil. Trans. R. Soc. Lond. A 339, 521–553 (1992).
Pfeifer, M. A., Williams, G. J., Vartanyants, I. A., Harder, R. & Robinson, I. K. Three-dimensional mapping of a deformation field inside a nanocrystal. Nature 442, 63–66 (2006).
Dierolf, M. et al. Ptychographic X-ray computed tomography at the nanoscale. Nature 467, 436–439 (2010).
Rodenburg, J. M. et al. Hard-X-ray lensless imaging of extended objects. Phys. Rev. Lett. 98, 034801 (2007).
Godard, P. et al. Three-dimensional high-resolution quantitative microscopy of extended crystals. Nat. Commun. 2, 568 (2011).
Berenguer, F. et al. X-ray lensless microscopy from undersampled diffraction intensities. Phys. Rev. B 88, 144101 (2013).
Pateras, A. I. et al. Nondestructive three-dimensional imaging of crystal strain and rotations in an extended bonded semiconductor heterostructure. Phys. Rev. B 92, 205305 (2015).
Birkbak, M. E., Guizar-Sicairos, M., Holler, M. & Birkedal, H. Internal structure of sponge glass fiber revealed by ptychographic nanotomography. J. Struct. Biol. 194, 124–128 (2016).
Holt, M. V. et al. Strain imaging of nanoscale semiconductor heterostructures with X-ray Bragg projection ptychography. Phys. Rev. Lett. 112, 165502 (2014).
Checa, A. G. et al. Crystallographic orientation inhomogeneity and crystal splitting in biogenic calcite. J. R. Soc. Interface 10, 20130425 (2013).
Godard, P., Allain, M., Chamard, V. & Rodenburg, J. Noise models for low counting rate coherent diffraction imaging. Opt. Express 20, 25914–25934 (2012).
Takagi, S. A dynamical theory of diffraction for a distorted crystal. J. Phys. Soc. Jpn 26, 1239–1253 (1969).
Hruszkewycz, S. O. et al. High-resolution three-dimensional structural microscopy by single-angle Bragg ptychography. Nat. Mater. 16, 244–251 (2017).
Nouet, J., Baronnet, A. & Howard, L. Crystallization in organo-mineral micro-domains in the crossed-lamellar layer of Nerita undata (Gastropoda, Neritopsina). Micron 43, 456–462 (2012).
Cuif, J. P. & Dauphin, Y. The Environment Recording Unit in coral skeletons—a synthesis of structural and chemical evidences for a biochemically driven, stepping-growth process in fibres. Biogeosciences 2, 61–73 (2005).
Bayerlein, B. et al. Self-similar mesostructure evolution of the growing mollusc shell reminiscent of thermodynamically driven grain growth. Nat. Mater. 13, 1102–1107 (2014).
Gower, L. B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 108, 4551–4627 (2008).
Xu, A.-W., Ma, Y. & Cölfen, H. Biomimetic mineralization. J. Mater. Chem. 17, 415–449 (2007).
Frølich, S. et al. Smaller calcite lattice deformation caused by occluded organic material in coccoliths than in mollusk shell. Cryst. Growth Des. 15, 2761–2767 (2015).
Li, H., Xin, H. L., Muller, D. A. & Estroff, L. A. Visualizing the 3D internal structure of calcite single crystals grown in agarose hydrogels. Science 326, 1244–1247 (2009).
Meldrum, F. C. & Coelfen, H. Crystallization and formation mechanisms of nanostructures. Nanoscale 2, 2326–2327 (2010).
Nudelman, F., Chen, H. H., Goldberg, H. A., Weiner, S. & Addadi, L. Spiers memorial lecture. Faraday Discuss. 136, 9–25 (2007).
Addadi, L., Moradian, J., Shay, E., Maroudas, N. G. & Weiner, S. A chemical model for the cooperation of sulfates and carboxylates in calcite crystal nucleation: relevance tor biomineralization. Proc. Natl Acad. Sci. USA 84, 2732–2736 (1987).
Gower, L. B. & Odon, D. J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 210, 719–734 (2000).
Bewernitz, M. A., Gebauer, D., Long, J., Cölfen, H. & Gower, L. B. A metastable liquid precursor phase of calcium carbonate and its interactions with polyaspartate. Faraday Discuss. 159, 291–312 (2012).
Acknowledgements
Y. Dauphin and J.-P. Cuif are warmly acknowledged for their invaluable inputs to the research programme. G. Le Moullac (IFREMER) is acknowledged for giving access to the shell samples. J. Savatier is warmly acknowledged for his help during the optical microscopy imaging session. We acknowledge the ESRF for providing access to the source. This work was funded by the French ANR under project number ANR-11-BS20-0005 and it has received funding from the European Research Council (ERC) under the European Union’s Horizon H2020 research and innovation programme grant agreement No 724881. Additional support was received from Aix-Marseille University A∗Midex (ANR-11-IDEX-0001-02), ANR grants France Bio Imaging (ANR-10-INSB-04-01) and France Life Imaging (ANR-11-INSB- 0006).
Author information
Authors and Affiliations
Contributions
V.C., J.Daillant and M.B. conceived the experiment with contributions from P.Godard, C.C. and P.Guenoun. P.Godard, M.A. and V.C. developed the inversion code. V.C., M.B., P.Godard, C.C. and J.Daillant performed the experiments at ID13, ESRF. The 3D ptychography reconstructions were performed by F.M. with inputs and discussion from V.C. and P.Godard. J.Duboisset performed the white light microscopy experiment and performed and analysed the CARS experiments. J.N. performed the AFM experiments. F.M., V.C., J.Daillant, C.C. and J.N. wrote the manuscript with contributions from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2538 kb)
Rights and permissions
About this article
Cite this article
Mastropietro, F., Godard, P., Burghammer, M. et al. Revealing crystalline domains in a mollusc shell single-crystalline prism. Nature Mater 16, 946–952 (2017). https://doi.org/10.1038/nmat4937
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4937
This article is cited by
-
Myriad Mapping of nanoscale minerals reveals calcium carbonate hemihydrate in forming nacre and coral biominerals
Nature Communications (2024)
-
First paleoproteome study of fossil fish otoliths and the pristine preservation of the biomineral crystal host
Scientific Reports (2023)
-
4th generation synchrotron source boosts crystalline imaging at the nanoscale
Light: Science & Applications (2022)
-
Revealing nano-scale lattice distortions in implanted material with 3D Bragg ptychography
Nature Communications (2021)