Abstract
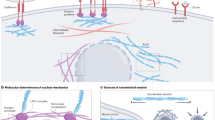
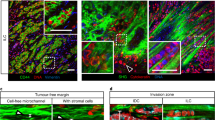
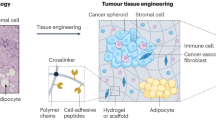
Within the heterogeneous architecture of tumour tissue there exists an elusive population of stem-like cells that are implicated in both recurrence and metastasis1,2. Here, by using engineered extracellular matrices, we show that geometric features at the perimeter of tumour tissue will prime a population of cells with a stem-cell-like phenotype. These cells show characteristics of cancer stem cells in vitro, as well as enhanced tumorigenicity in murine models of primary tumour growth and pulmonary metastases. We also show that interfacial geometry modulates cell shape, adhesion through integrin α5β1, MAPK and STAT activity, and initiation of pluripotency signalling. Our results for several human cancer cell lines suggest that interfacial geometry triggers a general mechanism for the regulation of cancer-cell state. Similar to how a growing tumour can co-opt normal soluble signalling pathways3, our findings demonstrate how cancer can also exploit geometry to orchestrate oncogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lu, P., Weaver, V. M. & Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 (2012).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature Rev. Cancer 8, 755–768 (2008).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Mehlen, P. & Puisieux, A. Metastasis: a question of life or death. Nature Rev. Cancer 6, 449–458 (2006).
Dean, M., Fojo, T. & Bates, S. Tumour stem cells and drug resistance. Nature Rev. Cancer 5, 275–284 (2005).
Magee, J. A., Piskounova, E. & Morrison, S. J. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell 21, 283–296 (2012).
Roesch, A. et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 141, 583–594 (2010).
Liu, J. et al. Soft fibrin gels promote selection and growth of tumorigenic cells. Nature Mater. 11, 734–741 (2012).
Tan, Y. et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nature Commun. 5, 4619 (2014).
Kumar, S. & Weaver, V. M. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 28, 113–127 (2009).
Discher, D. E., Janmey, P. & Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Swift, J. et al. Nuclear Lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013).
Ingber, D. E. Mechanical control of tissue growth: function follows form. Proc. Natl Acad. Sci. USA 102, 11571–11572 (2005).
Nelson, C. M. et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl Acad. Sci. USA 102, 11594–11599 (2005).
Friedl, P. & Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nature Rev. Mol. Cell Biol. 10, 445–457 (2009).
Nelson, C. M., Vanduijn, M. M., Inman, J. L., Fletcher, D. A. & Bissell, M. J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314, 298–300 (2006).
Murphy, W. L., Mcdevitt, T. C. & Engler, A. J. Materials as stem cell regulators. Nature Mater. 13, 547–557 (2014).
Gomez, E. W., Chen, Q. K., Gjorevski, N. & Nelson, C. M. Tissue geometry patterns epithelial–mesenchymal transition via intercellular mechanotransduction. J. Cell. Biochem. 110, 44–51 (2010).
Boghaert, E. et al. Host epithelial geometry regulates breast cancer cell invasiveness. Proc. Natl Acad. Sci. USA 109, 19632–19637 (2012).
Medema, J. P. Cancer stem cells: the challenges ahead. Nature Cell Biol. 15, 338–344 (2013).
Boiko, A. D. et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 466, 133–137 (2010).
Frank, N. Y. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 65, 4320–4333 (2005).
Sil, H., Sen, T. & Chatterjee, A. Fibronectin-integrin (α5β1) modulates migration and invasion of murine melanoma cell line B16F10 by involving MMP-9. Oncol. Res. 19, 335–348 (2011).
Manning, M. L., Foty, R. A., Steinberg, M. S. & Schoetz, E. M. Coaction of intercellular adhesion and cortical tension specifies tissue surface tension. Proc. Natl Acad. Sci. USA 107, 12517–12522 (2010).
Roux, P. P. & Blenis, J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344 (2004).
Nicholas, C. & Lesinski, G. B. in The Jak-STAT signal transduction pathway in melanoma, 283–306 (Breakthroughs in Melanoma Research, 2011).
Lee, J., Abdeen, A. A. & Kilian, K. A. Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment. Sci. Rep. 4, 5188 (2014).
Lecuit, T. & Lenne, P. F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nature Rev. Mol. Cell Biol. 8, 633–644 (2007).
Kollmannsberger, P., Bidan, C. M., Dunlop, J. W. C. & Fratzl, P. The physics of tissue patterning and extracellular matrix organisation: how cells join forces. Soft Matter 7, 9549–9560 (2011).
Hegerfeldt, Y., Tusch, M., Bröcker, E. B. & Friedl, P. Collective cell movement in primary melanoma explants. Cancer Res. 62, 2125–2130 (2002).
Lee, J., Abdeen, A. A., Kim, A. & Kilian, K. A. The influence of biophysical parameters on maintaining the mesenchymal stem cell phenotype. ACS Biomater. Sci. Eng. 1, 218–226 (2015).
Tse, J. R. & Engler, A. J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 47, 10.16.1–10.16.16 (2010).
Damljanović, V., Lagerholm, B. C. & Jacobson, K. Bulk and micropatterned conjugation of extracellular matrix proteins to characterized polyacrylamide substrates for cell mechanotransduction assays. Biotechniques 39, 847–851 (2005).
Chang, C.-W., van Spreeuwel, A., Zhang, C. & Varghese, S. PEG/clay nanocomposite hydrogel: a mechanically robust tissue engineering scaffold. Soft Matter 6, 5157–5164 (2010).
Miller, J. S. et al. Bioactive hydrogels made from step-growth derived PEG-peptide macromers. Biomaterials 31, 3736–3743 (2010).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675 (2012).
Zhang, D. & Kilian, K. A. The effect of mesenchymal stem cell shape on the maintenance of multipotency. Biomaterials 34, 3962–3969 (2013).
Reich, M. et al. GenePattern 2.0. Nature Genet. 38, 500–501 (2006).
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 30–33 (2000).
Acknowledgements
This research was supported with funding from the American Cancer Society Illinois Division Grant # 281225 and the National Science Foundation Grant # 1454616 CAR. Graduate student (K.L.W.) support was provided by Morris Animal Foundation. We thank the Beckman Institute ITG facilities, Institute of Genomic Biology Imaging facilities, Micro and Nanotechnology Laboratory facilities, and the Roy J. Carver Biotechnology Center.
Author information
Authors and Affiliations
Contributions
J.L. and K.A.K. conceived the ideas and designed the experiments. J.L., A.A.A., K.L.W. and T.M.F. conducted the experiments. J.L., A.A.A., K.L.W., T.M.F. and K.A.K. analysed the data. J.L., A.A.A., T.M.F. and K.A.K. interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 5317 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Abdeen, A., Wycislo, K. et al. Interfacial geometry dictates cancer cell tumorigenicity. Nature Mater 15, 856–862 (2016). https://doi.org/10.1038/nmat4610
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4610
This article is cited by
-
How multiscale curvature couples forces to cellular functions
Nature Reviews Physics (2024)
-
Cell–extracellular matrix mechanotransduction in 3D
Nature Reviews Molecular Cell Biology (2023)
-
The ECM and tissue architecture are major determinants of early invasion mediated by E-cadherin dysfunction
Communications Biology (2023)
-
Usability of deep learning and H&E images predict disease outcome-emerging tool to optimize clinical trials
npj Precision Oncology (2022)
-
Lessons from the Embryo: an Unrejected Transplant and a Benign Tumor
Stem Cell Reviews and Reports (2021)