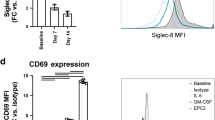
Abstract
Eotaxin is an eosinophil–specific chemoattractant that has been recently identified in rodent models of asthma and host response against tumors. To determine whether a similar molecule might play a role in human inflammatory diseases characterized by eosinophilia, we isolated the human eotaxin gene. We demonstrate that human eotaxin is an early response gene of cytokine–stimulated epithelial and endothelial cells, and is induced in peripheral blood eosinophils by interleukin–3. Eotaxin is directly chemotactic for eosinophils, but not mononuclear cells or neutrophils. Eotaxin messenger RNA accumulates markedly in the lesions of patients with inflammatory bowel disease (ulcerative colitis and Crohn's disease), but not in the lesions of patients with diverticulitis. These results now provide a mechanism involving eotaxin to explain the eosinophil infiltration seen in a variety of human diseases; as such, an eotaxin antagonist may be a novel therapy for certain human diseases characterized by tissue eosinophilia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gleich, G.J. & Adolphson, C.R. The eosinophilic leukocyte: Structure and function. Adv. Immunol. 39, 177–253 (1986).
Weller, P.P. The immunobiology of eosinophils. N. Engl. J. Med. 324, 1110–1118 (1991).
Bousquett, J. et al. Eosinophilic inflammation in asthma. N. Engl. J. Med. 323, 1033–1039 (1990).
Butcher, E.G. Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell 67, 1033–1036 (1991).
Baggiolini, M., Dewald, B. & Moser, B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv. Immunol. 55, 97–179 (1994).
Murphy, P.M. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 12, 593–633 (1994).
Jose, P.J. et al. Eotaxin: A potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J. Exp. Med. 179, 881–887 (1994).
Jose, P.J. et al. Eotaxin: Cloning of an eosinophils chemoattractant cytokine and increased mRNA expression in allergen-challenged guinea-pig lungs. Biochem. Biophys. Res. Commun. 205, 788–794 (1994).
Rothenberg, M.E., Luster, A.D., Lilly, C.M., Drazin, J.M. & Leder, P. Constitutive and allergen-induced expression of eotaxin mRNA in the guinea pig lung. J. Exp. Med. 181, 1211–1216 (1994).
Griffiths-Johnson, D.A., Collins, P.D., Rossi, A.G., Jose, P.J. & Williams, T.J. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro and causes their accumulation into the lung in vivo. Biochem. Biophys. Res. Commun. 197, 1167–1172 (1993).
Rothenberg, M.E., Luster, A.D. & Leder, P. Murine eotaxin: An eosinophil chemoattractant inducible in endothelial cells and in IL-4-induced tumor suppression. Proc. Natl Acad. Sci. USA 92, 8960–8964 (1995).
Zhang, Y.J., Rutledge, J. & Rollins, B. Structure/activity analysis of human monocyte chemoattractant protein-l(MCP-l) by mutagenesis. J. Biol Chem. 269, 15918–15924 (1994).
Gong, J.-H. & Clark-Lewis, I. Antagonists of monocyte chemoattractant protein 1 identified by modification of functionally critical NH2-terminal residues. J. Exp. Med. 181, 631–640 (1995).
Shaw, G. & Kamen, R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46, 659–667 (1986).
Jiang, Y., Valente, A.J., Williamson, M.J., Zhang, L. & Graves, D.T. Post-translational modification of a monocyte-specific chemoattractant synthesized by glioma, osteosarcoma, and vascular smooth muscle cells. J. Biol Chem. 265, 18318–18321 (1990).
Rothenberg, M.E. et al. Eosinophils cocultured with endothelial cells have increased survival and functional properties. Science 237, 645–647 (1987).
Cromwell, O. et al. Expression and generation of interleukin-8, interleukin-6 and granulocyte-macrophage colony-stimulating factor by bronchial cells and enhancement of IL-lβ and tumor necrosis factor α. Immunology 77, 330 (1992).
Spry, C.J. Mechanism of eosinophilia. V. Kinetics of normal and accelerated eosinophilpoiesis. Cell Tissue Kinet. 4, 351–364 (1971).
Mosmann, T.R. & Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989).
Buckley, M.G. et al. IL-4 enhances IL-3 and IL-8 gene expression in a human leukemic mast cell line. Immunology 84, 410–415 (1995).
Kita, H. H. et al. Granulocyte/macrophage colony-stimulating factor and inter-leukin 3 release from human peripheral blood eosinophils and neutrophils. J. Exp. Med. 174, 745–748 (1991).
Rothenberg, M.E. et al. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human inter-leukin 3. J. Clin. Invest. 81, 1986–1992 (1988).
Fuggle, W.J., Crocker, J. & Smith, P.J. A quantitative study of eosinophil poly-morphs in Hodgkin's disease. J. Clin. Pathol. 37, 267–271 (1984).
Walsh, R.E. & Gaginella, T.S. The eosinophil in inflammatory bowel disease. Scand. J. Gastroenterol. 26, 1217–1224 (1991).
Sarin, S.K. et al. Significance of eosinophil and mast cell counts in rectal mucosa in ulcerative colitis: A prospective controlled study. Dig. Dis. Sci. 32, 363–367 (1978).
Willoughby, C.P., Piris, J. & Truelove, S.C. Tissue eosinophils in ulcerative colitis. Scand. J. Gastroenterol. 14, 395–399 (1979).
Dvorak, A.M. Ultrastructural evidence for release of major basic protein-containing crystalline cores of eosinophil granules in vivo: Cytotoxic potential in Crohn's disease. J. Immunol. 125, 460–462 (1980).
Hallgren, R. et al. Neutrophil and eosinophil involvement of the small bowel in patients with celiac disease and Crohn's disease: Studies on the secretion rate and immunohistochemical localization of granulocyte granule constituents. Am. J. Med. 86, 56–64 (1989).
Dubucquoi, S. et al. Activated eosinophils and interleukin 5 expression in early recurrence of Crohn's disease. Gut 37, 242–246 (1995).
Collins, P.O., Marleau, S., Griffiths-Johnson, D.A., Jose, P.J. & Williams, T.J. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 182, 1169–1174 (1995).
Powrie, F. T cells and inflammatory bowel disease: Protective and pathogenic role. Immunity 3, 171–174 (1995).
Tomonaga, M., Gasson, J.C., Quan, S.G. & Golde, D.W. Establishment of eosinophilic sublines from human promyelocytic leukemia (HL-60) cells: Demonstration of multipotentiality and single-lineage commitment of HL-60 stem cells. Blood 67, 1433–1441 (1986).
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. Basic local alignment search tool. J. Mol Biol. 215, 403–410 (1990).
Seed, B. & Aruffo, A. Molecular cloning of the CD2 antigen, the T-cell erythro-cyte receptor, by a rapid immunoselection procedure. Proc. Natl. Acad. Sci. USA 84, 3365–3369 (1987).
Hansel, T.T. et al. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J. Immunol. Methods 145, 105–110 (1991).
Falk, W., Goodwin, R.H. & Leonard, E.J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J. Immunol. Methods 33, 239–247 (1980).
Cybulsky, M.I. et al. Alternative splicing of human VCAM-1 in activated vascular endothelium. Am. J. Pathol. 138, 815–820 (1991).
Birnstiel, M.L. & Busslinger, M. Transcription termination and 3′ processing: The end is in site! Cell 41, 349–359 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garcia-Zepeda, E., Rothenberg, M., Ownbey, R. et al. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med 2, 449–456 (1996). https://doi.org/10.1038/nm0496-449
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0496-449
This article is cited by
-
Immune and non-immune functions of adipose tissue leukocytes
Nature Reviews Immunology (2022)
-
Asthma-related inflammation promotes lung metastasis of breast cancer cells through CCL11–CCR3 pathway
Respiratory Research (2021)
-
Chemokines orchestrate tumor cells and the microenvironment to achieve metastatic heterogeneity
Cancer and Metastasis Reviews (2021)
-
CCL2 is associated with microglia and macrophage recruitment in chronic traumatic encephalopathy
Journal of Neuroinflammation (2020)
-
Renal expression of cytokines and chemokines in diabetic nephropathy
BMC Nephrology (2020)