Abstract
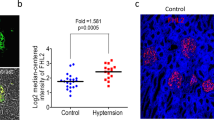
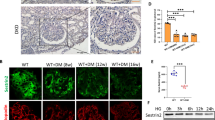
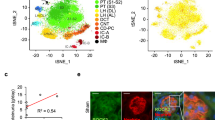
In chronic kidney disease (CKD), loss of functional nephrons results in metabolic and mechanical stress in the remaining ones, resulting in further nephron loss. Here we show that Akt2 activation has an essential role in podocyte protection after nephron reduction. Glomerulosclerosis and albuminuria were substantially worsened in Akt2−/− but not in Akt1−/− mice as compared to wild-type mice. Specific deletion of Akt2 or its regulator Rictor in podocytes revealed that Akt2 has an intrinsic function in podocytes. Mechanistically, Akt2 triggers a compensatory program that involves mouse double minute 2 homolog (Mdm2), glycogen synthase kinase 3 (Gsk3) and Rac1. The defective activation of this pathway after nephron reduction leads to apoptosis and foot process effacement of the podocytes. We further show that AKT2 activation by mammalian target of rapamycin complex 2 (mTORC2) is also required for podocyte survival in human CKD. More notably, we elucidate the events underlying the adverse renal effect of sirolimus and provide a criterion for the rational use of this drug. Thus, our results disclose a new function of Akt2 and identify a potential therapeutic target for preserving glomerular function in CKD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
US Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, 2010).
Mahmoodi, B.K. et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 380, 1649–1661 (2012).
Hostetter, T.H. Progression of renal disease and renal hypertrophy. Annu. Rev. Physiol. 57, 263–278 (1995).
Hostetter, T.H. Hyperfiltration and glomerulosclerosis. Semin. Nephrol. 23, 194–199 (2003).
Kriz, W. & LeHir, M. Pathways to nephron loss starting from glomerular diseases—insights from animal models. Kidney Int. 67, 404–419 (2005).
Franke, T.F. Intracellular signaling by Akt: bound to be specific. Sci. Signal. 1, pe29 (2008).
Ceci, M., Ross, J. Jr. & Condorelli, G. Molecular determinants of the physiological adaptation to stress in the cardiomyocyte: a focus on AKT. J. Mol. Cell. Cardiol. 37, 905–912 (2004).
Manning, B.D. & Cantley, L.C. AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 (2007).
Shankland, S.J. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 69, 2131–2147 (2006).
Mundel, P. & Reiser, J. Proteinuria: an enzymatic disease of the podocyte? Kidney Int. 77, 571–580 (2010).
Zhu, J. et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 73, 556–566 (2008).
Huber, T.B. et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol. Cell. Biol. 23, 4917–4928 (2003).
Chuang, P.Y. & He, J.C. Signaling in regulation of podocyte phenotypes. Nephron Physiol. 111, p9–p15 (2009).
Tejada, T. et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 73, 1385–1393 (2008).
Stylianou, K. et al. The PI3K/Akt/mTOR pathway is activated in murine lupus nephritis and downregulated by rapamycin. Nephrol. Dial. Transplant. 26, 498–508 (2011).
Laouari, D. et al. TGF-α mediates genetic susceptibility to chronic kidney disease. J. Am. Soc. Nephrol. 22, 327–335 (2011).
Tschopp, O. et al. Essential role of protein kinase Bγ (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132, 2943–2954 (2005).
Yumura, W. et al. Age-associated changes in renal glomeruli of mice. Exp. Gerontol. 24, 237–249 (1989).
Cho, H. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292, 1728–1731 (2001).
Garofalo, R.S. et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. J. Clin. Invest. 112, 197–208 (2003).
Shibata, S. et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat. Med. 14, 1370–1376 (2008).
Akilesh, S. et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J. Clin. Invest. 121, 4127–4137 (2011).
Scott, R.P. et al. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J. Am. Soc. Nephrol. 23, 1149–1154 (2012).
Faul, C. et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14, 931–938 (2008).
Sarbassov, D.D. et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 (2004).
Feng, J., Park, J., Cron, P., Hess, D. & Hemmings, B.A. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279, 41189–41196 (2004).
Gödel, M. et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 121, 2197–2209 (2011).
Kreis, H. et al. Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J. Am. Soc. Nephrol. 15, 809–817 (2004).
Letavernier, E., Pe'raldi, M.N., Pariente, A., Morelon, E. & Legendre, C. Proteinuria following a switch from calcineurin inhibitors to sirolimus. Transplantation 80, 1198–1203 (2005).
Niranjan, T. et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat. Med. 14, 290–298 (2008).
Wei, C. et al. Modification of kidney barrier function by the urokinase receptor. Nat. Med. 14, 55–63 (2008).
Reiser, J. et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J. Clin. Invest. 113, 1390–1397 (2004).
Dai, C. et al. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 20, 1997–2008 (2009).
Ma, H. et al. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J. Am. Soc. Nephrol. 21, 1145–1156 (2010).
Faour, W.H., Thibodeau, J.F. & Kennedy, C.R. Mechanical stretch and prostaglandin E2 modulate critical signaling pathways in mouse podocytes. Cell. Signal. 22, 1222–1230 (2010).
Faul, C., Asanuma, K., Yanagida-Asanuma, E., Kim, K. & Mundel, P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 17, 428–437 (2007).
Reiser, J. et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and α3 integrin. J. Biol. Chem. 279, 34827–34832 (2004).
Yanagida-Asanuma, E. et al. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am. J. Pathol. 171, 415–427 (2007).
Zhou, G.L. et al. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J. Biol. Chem. 281, 36443–36453 (2006).
Welsh, G.I. et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 12, 329–340 (2010).
Letavernier, E. et al. Sirolimus interacts with pathways essential for podocyte integrity. Nephrol. Dial. Transplant. 24, 630–638 (2009).
Sarbassov, D.D. et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 (2006).
Vollenbröker, B. et al. mTOR regulates expression of slit diaphragm proteins and cytoskeleton structure in podocytes. Am. J. Physiol. Renal Physiol. 296, F418–F426 (2009).
Lamming, D.W. et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 (2012).
Inoki, K. et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 121, 2181–2196 (2011).
Rangan, G.K. & Coombes, J.D. Renoprotective effects of sirolimus in non-immune initiated focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 22, 2175–2182 (2007).
Bonegio, R.G. et al. Rapamycin ameliorates proteinuria-associated tubulointerstitial inflammation and fibrosis in experimental membranous nephropathy. J. Am. Soc. Nephrol. 16, 2063–2072 (2005).
Naumovic, R. et al. Effects of rapamycin on active Heymann nephritis. Am. J. Nephrol. 27, 379–389 (2007).
Ito, N. et al. mTORC1 activation triggers the unfolded protein response in podocytes and leads to nephrotic syndrome. Lab. Invest. 91, 1584–1595 (2011).
Diekmann, F. et al. Mammalian target of rapamycin inhibition halts the progression of proteinuria in a rat model of reduced renal mass. J. Am. Soc. Nephrol. 18, 2653–2660 (2007).
Lindsley, C.W., Barnett, S.F., Layton, M.E. & Bilodeau, M.T. The PI3K/Akt pathway: recent progress in the development of ATP-competitive and allosteric Akt kinase inhibitors. Curr. Cancer Drug Targets 8, 7–18 (2008).
Cho, H., Thorvaldsen, J.L., Chu, Q., Feng, F. & Birnbaum, M.J. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276, 38349–38352 (2001).
Lu, M. et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat. Med. 18, 388–395 (2012).
Moeller, M.J., Sanden, S.K., Soofi, A., Wiggins, R.C. & Holzman, L.B. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35, 39–42 (2003).
Terzi, F. et al. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J. Clin. Invest. 106, 225–234 (2000).
Shankland, S.J., Pippin, J.W., Reiser, J. & Mundel, P. Podocytes in culture: past, present, and future. Kidney Int. 72, 26–36 (2007).
Pillebout, E. et al. Proliferation and remodeling of the peritubular microcirculation after nephron reduction: association with the progression of renal lesions. Am. J. Pathol. 159, 547–560 (2001).
Viau, A. et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J. Clin. Invest. 120, 4065–4076 (2010).
Solez, K. et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am. J. Transplant. 8, 753–760 (2008).
Mollet, G. et al. Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J. Am. Soc. Nephrol. 20, 2181–2189 (2009).
Lautrette, A. et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat. Med. 11, 867–874 (2005).
Acknowledgements
We thank L.H. Noël and the clinicians of the Renal Transplant Department of Necker hospital for patient data and sample collection. We are grateful to M.J. Birnbaum (University of Pennsylvania) and M. Hall (Basel University) for Akt and Rictor mutant mice, respectively, C. Antignac (INSERM U983) for podocin-specific antibodies and R. Montjean (INSERM U983) for the PAK1-GST fusion protein expression vector. We thank M.C. Gubler, C. Antignac, C. Sumida and S. Harvey for critical advice. This work was supported by INSERM, Université Paris Descartes, Assistance Publique–Hôpitaux de Paris (AP-HP), Agence National pour la Recherche (to F.T. and M. Pontoglio), Fondation de la Recherche Médicale (to F.T. and M. Pontoglio), Association pour l'Information et la Recherche sur les Maladies Rénales Génétiques, Association pour l'Utilisation du rein Artificiel, Roche Laboratories, the Excellence Initiative of the German Federal and State Governments EXC 294 (to T.B.H.) and European Community's Seventh Framework Program FP7/2009 - agreement number 241955, SYSCILIA (to M. Pontoglio).
Author information
Authors and Affiliations
Contributions
G.C. and F.B. designed and performed the experiments, analyzed the data and wrote the paper. A.V. and C.T. also performed some experiments and analyzed the data. W.B. performed the in vitro studies. C.N., M.B. and S.B. performed the mouse experiments (breeding, surgery and morphological studies). K.G. and A.O.M. performed the electron microscopy studies. S.Z. and T.B.H. provided the RictorΔpod mice and were involved in data analysis. C.L. provided and characterized patient samples and clinical outcome data. G.F., M. Pontoglio and M. Pende were involved in data analysis and wrote the paper. F.T. provided the conceptual framework and designed the study, supervised the project and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1–2 (PDF 6133 kb)
Rights and permissions
About this article
Cite this article
Canaud, G., Bienaimé, F., Viau, A. et al. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19, 1288–1296 (2013). https://doi.org/10.1038/nm.3313
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3313
This article is cited by
-
A preliminary study of the miRNA restitution effect on CNV-induced miRNA downregulation in CAKUT
BMC Genomics (2024)
-
Nutrient-sensing mTORC1 and AMPK pathways in chronic kidney diseases
Nature Reviews Nephrology (2023)
-
The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity
Military Medical Research (2022)
-
Deficiency of the Src homology phosphatase 2 in podocytes is associated with renoprotective effects in mice under hyperglycemia
Cellular and Molecular Life Sciences (2022)
-
The ability of remaining glomerular podocytes to adapt to the loss of their neighbours decreases with age
Cell and Tissue Research (2022)