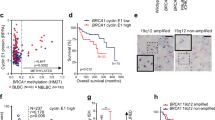
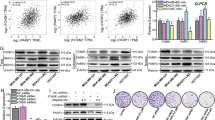
Abstract
Cells that are deficient in homologous recombination, such as those that lack functional breast cancer–associated 1 (BRCA1) or BRCA2, are hypersensitive to inhibition of poly(ADP-ribose) polymerase (PARP). However, BRCA-deficient tumors represent only a small fraction of adult cancers, which might restrict the therapeutic utility of PARP inhibitor monotherapy. Cyclin-dependent kinase 1 (Cdk1) phosphorylates BRCA1, and this is essential for efficient formation of BRCA1 foci. Here we show that depletion or inhibition of Cdk1 compromises the ability of cells to repair DNA by homologous recombination. Combined inhibition of Cdk1 and PARP in BRCA–wild-type cancer cells resulted in reduced colony formation, delayed growth of human tumor xenografts and tumor regression with prolonged survival in a mouse model of lung adenocarcinoma. Inhibition of Cdk1 did not sensitize nontransformed cells or tissues to inhibition of PARP. Because reduced Cdk1 activity impaired BRCA1 function and consequently, repair by homologous recombination, inhibition of Cdk1 represents a plausible strategy for expanding the utility of PARP inhibitors to BRCA-proficient cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Shapiro, G.I. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 24, 1770–1783 (2006).
Malumbres, M. & Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9, 153–166 (2009).
Hochegger, H. et al. An essential role for Cdk1 in S phase control is revealed via chemical genetics in vertebrate cells. J. Cell Biol. 178, 257–268 (2007).
Ira, G. et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431, 1011–1017 (2004).
Jazayeri, A. et al. ATM- and cell cycle–dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 8, 37–45 (2006).
Tian, B., Yang, Q. & Mao, Z. Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat. Cell Biol. 11, 211–218 (2009).
Myers, J.S., Zhao, R., Xu, X., Ham, A.J. & Cortez, D. Cyclin-dependent kinase 2 dependent phosphorylation of ATRIP regulates the G2-M checkpoint response to DNA damage. Cancer Res. 67, 6685–6690 (2007).
Johnson, N. et al. Cdk1 participates in BRCA1-dependent S phase checkpoint control in response to DNA damage. Mol. Cell 35, 327–339 (2009).
Moynahan, M.E., Chiu, J.W., Koller, B.H. & Jasin, M. Brca1 controls homology-directed DNA repair. Mol. Cell 4, 511–518 (1999).
Bryant, H.E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
McCabe, N. et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 66, 8109–8115 (2006).
Ashworth, A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 26, 3785–3790 (2008).
Fong, P.C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Tutt, A. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376, 235–244 (2010).
Audeh, M.W. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376, 245–251 (2010).
Turner, N., Tutt, A. & Ashworth, A. Hallmarks of 'BRCAness' in sporadic cancers. Nat. Rev. Cancer 4, 814–819 (2004).
Bhattacharyya, A., Ear, U.S., Koller, B.H., Weichselbaum, R.R. & Bishop, D.K. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J. Biol. Chem. 275, 23899–23903 (2000).
Elstrodt, F. et al. BRCA1 mutation analysis of 41 human breast cancer cell lines reveals three new deleterious mutants. Cancer Res. 66, 41–45 (2006).
Cai, D., Latham, V.M. Jr., Zhang, X. & Shapiro, G.I. Combined depletion of cell cycle and transcriptional cyclin-dependent kinase activities induces apoptosis in cancer cells. Cancer Res. 66, 9270–9280 (2006).
Vassilev, L.T. et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. USA 103, 10660–10665 (2006).
Pierce, A.J., Johnson, R.D., Thompson, L.H. & Jasin, M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13, 2633–2638 (1999).
Brown, A.P. et al. Toxicity and toxicokinetics of the cyclin-dependent kinase inhibitor AG-024322 in cynomolgus monkeys following intravenous infusion. Cancer Chemother. Pharmacol. 62, 1091–1101 (2008).
Calabrese, C.R. et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J. Natl. Cancer Inst. 96, 56–67 (2004).
Thomas, H.D. et al. Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol. Cancer Ther. 6, 945–956 (2007).
Plummer, R. et al. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin. Cancer Res. 14, 7917–7923 (2008).
Hackett, A.J. et al. Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines. J. Natl. Cancer Inst. 58, 1795–1806 (1977).
Ji, H. et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 448, 807–810 (2007).
Turner, N.C. et al. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 27, 1368–1377 (2008).
Esashi, F. et al. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 434, 598–604 (2005).
Jackson, E.L. et al. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 65, 10280–10288 (2005).
Oliver, T.G. et al. Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer. Genes Dev. 24, 837–852 (2010).
Herbst, R.S., Heymach, J.V. & Lippman, S.M. Lung cancer. N. Engl. J. Med. 359, 1367–1380 (2008).
Santamaría, D. et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 448, 811–815 (2007).
Garcia-Higuera, I. et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol. Cell 7, 249–262 (2001).
Ji, H. et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell 9, 485–495 (2006).
Acknowledgements
This work was supported by US National Institutes of Health (NIH) Grants R01 CA090687 (G.I.S.), P50 CA089393 (Dana-Farber/Harvard Cancer Center (DF/HCC) Specialized Program of Research Excellence (SPORE) in Breast Cancer), including Developmental Project Funding (G.I.S.) and a Career Development Award (N.J.), as well as Susan G. Komen Post-Doctoral Fellowship Award KG080773 (N.J., G.I.S. and N.J.C.) and NIH Grant P50 CA090578 (DF/HCC SPORE in Lung Cancer) (G.I.S. and K.-K.W.). H.D.T., N.J.C. and D.R.N. were supported by a Programme Grant from Cancer Research U.K. K.-K.W. was also supported by a Uniting Against Lung Cancer grant and NIH grants U01 CA141576, R01 AG2400401, R01 CA122794, R01 CA140594 and 1RC2 CA147940-01. We thank M. Arora and J.D. Parvin for assistance with preliminary experiments; D. Skinner for technical assistance with tissue block and slide preparation; members of the Confocal and Light Microscopy Core Facility at the Dana-Farber Cancer Institute for technical assistance with acquisition of confocal microscopy images; M. Jasin (Memorial Sloan-Kettering Cancer Center) for U2OS pDR-GFP cells; and K. Hook, D. Madren and Z. Hostomsky of Pfizer for AG14361, AG014699 (PF-01367338) and AG024322 (PF-00176275).
Author information
Authors and Affiliations
Contributions
N.J. and G.I.S. designed this study. N.J., Y-C.L., L.A.M., D.L., Z.E.W., K.A.C., C.U., R.T.B. and H.D.T. performed the experiments. N.J. and G.I.S. analyzed the data. N.J. and G.I.S. communicated with S.J.R., K.-K.W., D.R.N., A.D.d'A. and N.J.C. about the data interpretation and wrote the manuscript. G.I.S. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Methods (PDF 941 kb)
Rights and permissions
About this article
Cite this article
Johnson, N., Li, YC., Walton, Z. et al. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat Med 17, 875–882 (2011). https://doi.org/10.1038/nm.2377
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2377
This article is cited by
-
LCK facilitates DNA damage repair by stabilizing RAD51 and BRCA1 in the nucleus of chemoresistant ovarian cancer
Journal of Ovarian Research (2023)
-
miR-195-5p Targets CDK1 To Regulate New DNA Synthesis and Inhibit the Proliferation of Hepatocellular Carcinoma Cells
Applied Biochemistry and Biotechnology (2023)
-
Pilot clinical trial and phenotypic analysis in chemotherapy-pretreated, metastatic triple-negative breast cancer patients treated with oral TAK-228 and TAK-117 (PIKTOR) to increase DNA damage repair deficiency followed by cisplatin and nab paclitaxel
Biomarker Research (2023)
-
PBK drives PARP inhibitor resistance through the TRIM37/NFκB axis in ovarian cancer
Experimental & Molecular Medicine (2022)
-
Mass spectrometry-based draft of the mouse proteome
Nature Methods (2022)