Abstract
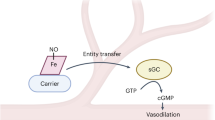
Control of blood vessel tone is central to vascular homeostasis. Here we show that metabolism of tryptophan to kynurenine by indoleamine 2,3-dioxygenase (Ido) expressed in endothelial cells contributes to arterial vessel relaxation and the control of blood pressure. Infection of mice with malarial parasites (Plasmodium berghei) or induction of endotoxemia in mice led to endothelial expression of Ido, decreased plasma tryptophan concentration, increased kynurenine concentration and hypotension. Pharmacological inhibition of Ido increased blood pressure in systemically inflamed mice but not in mice deficient in Ido or interferon-γ, which is required for Ido induction. Both tryptophan and kynurenine dilated preconstricted porcine coronary arteries; the dilating effect of tryptophan required the presence of active Ido and an intact endothelium, whereas the effect of kynurenine was endothelium independent. The arterial relaxation induced by kynurenine was mediated by activation of the adenylate and soluble guanylate cyclase pathways. Kynurenine administration decreased blood pressure in a dose-dependent manner in spontaneously hypertensive rats. Our results identify tryptophan metabolism by Ido as a new pathway contributing to the regulation of vascular tone.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
06 May 2010
In the version of this article initially published, the symbol key in Figure 2g,h is incorrect. The correct symbol key is open circles for Ido1–/– and filled circles for WT. The error has been corrected in the HTML and PDF versions of the article.
References
Riedemann, N.C., Guo, R.F. & Ward, P.A. Novel strategies for the treatment of sepsis. Nat. Med. 9, 517–524 (2003).
Gómez-Jiménez, J. et al. L-arginine: nitric oxide pathway in endotoxemia and human septic shock. Crit. Care Med. 23, 253–258 (1995).
Ignarro, L.J., Cirino, G., Casini, A. & Napoli, C. Nitric oxide as a signaling molecule in the vascular system: an overview. J. Cardiovasc. Pharmacol. 34, 879–886 (1999).
Kröncke, K.D., Fehsel, K. & Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 113, 147–156 (1998).
Meyer, J. et al. Reversal of hyperdynamic response to continuous endotoxin administration by inhibition of NO synthesis. J. Appl. Physiol. 73, 324–328 (1992).
MacMicking, J.D. et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 641–650 (1995).
Ochoa, J.B. et al. Nitrogen oxide levels in patients after trauma and during sepsis. Ann. Surg. 214, 621–626 (1991).
Bakker, J. et al. Administration of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) by intravenous infusion for up to 72 hours can promote the resolution of shock in patients with severe sepsis: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144–002). Crit. Care Med. 32, 1–12 (2004).
Kirkebøen, K.A. & Strand, O.A. The role of nitric oxide in sepsis—an overview. Acta Anaesthesiol. Scand. 43, 275–288 (1999).
Cauwels, A., Janssen, B., Buys, E., Sips, P. & Brouckaert, P. Anaphylactic shock depends on PI3K and eNOS-derived NO. J. Clin. Invest. 116, 2244–2251 (2006).
Thomas, S.R. & Stocker, R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 4, 199–220 (1999).
Yoshida, R., Oku, T., Kishida, T. & Hayaishi, O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc. Natl. Acad. Sci. USA 78, 129–132 (1981).
Werner, E.R. et al. Interferon-γ–induced degradation of tryptophan by human cells in vitro. Biol. Chem. Hoppe Seyler 368, 1407–1412 (1987).
Hansen, A.M., Driussi, C., Turner, V., Takikawa, O. & Hunt, N.H. Tissue distribution of indoleamine 2,3-dioxygenase in normal and malaria-infected tissue. Redox Rep. 5, 112–115 (2000).
Ball, H.J., McParland, B., Driussi, C. & Hunt, N.H. Isolating vessels from the mouse brain for gene expression analysis using laser capture microdissection. Brain Res. Brain Res. Protoc. 9, 206–213 (2002).
Sakash, J.B., Byrne, G.I., Lichtman, A. & Libby, P. Cytokines induce indoleamine 2,3-dioxygenase expression in human atheroma-associated cells: implications for persistent Chlamydophila pneumoniae infection. Infect. Immun. 70, 3959–3961 (2002).
Owe-Young, R. et al. Kynurenine pathway metabolism in human blood-brain barrier cells: implications for immune tolerance and neurotoxicity. J. Neurochem. 105, 1346–1357 (2008).
Mitchell, A.J. et al. Early cytokine production is associated with protection from murine cerebral malaria. Infect. Immun. 73, 5645–5653 (2005).
Hansen, A.M. et al. Increased expression of indoleamine 2,3-dioxygenase in murine malaria infection is predominantly localised to the vascular endothelium. Int. J. Parasitol. 34, 1309–1319 (2004).
Bruneel, F. et al. Shock complicating severe falciparum malaria in European adults. Intensive Care Med. 23, 698–701 (1997).
Chang, W.L. et al. CD8+ T cell depletion ameliorates circulatory shock in Plasmodium berghei–infected mice. Infect. Immun. 69, 7341–7348 (2001).
Munn, D.H. et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189, 1363–1372 (1999).
Connelly, L., Madhani, M. & Hobbs, A.J. Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice: a pro-inflammatory role for eNOS-derived NO in vivo. J. Biol. Chem. 280, 10040–10046 (2005).
Quaschning, T. et al. Lack of endothelial nitric oxide synthase promotes endothelin-induced hypertension: lessons from endothelin-1 transgenic/endothelial nitric oxide synthase knockout mice. J. Am. Soc. Nephrol. 18, 730–740 (2007).
Neill, A.L. & Hunt, N.H. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology 105, 165–175 (1992).
Sanni, L.A. et al. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. Am. J. Pathol. 152, 611–619 (1998).
Stasch, J.P. et al. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br. J. Pharmacol. 136, 773–783 (2002).
Schmidt, H.H.H.W., Schmidt, P.M. & Stasch, J.P. NO- and haem-independent soluble guanylate cyclase activators. in Handbood of Experimental Pharmacology: cGMP Generators, Effectors and Therapeutic Implications (eds. Hofmann, F., Schmidt, H.H.H.W. & Stasch, J.P.) 309–339 (Springer Verlag, Berlin, 2009).
Stasch, J.P. et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J. Clin. Invest. 116, 2552–2561 (2006).
Butt, E. et al. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J. Biol. Chem. 269, 14509–14517 (1994).
Lincoln, T.M., Cornwell, T.L. & Taylor, A.E. cGMP-dependent protein kinase mediates the reduction of Ca2+ by cAMP in vascular smooth muscle cells. Am. J. Physiol. 258, C399–C407 (1990).
Armstrong, R.S., Irwin, M.J. & Wright, P.E. Resonance Raman evidence for constrained heme structure in soybean leghemoglobin and its derivatives. Biochem. Biophys. Res. Commun. 95, 682–689 (1980).
Jung, I.D. et al. Blockade of indoleamine 2,3-dioxygenase protects mice against lipopolysaccharide-induced endotoxin shock. J. Immunol. 182, 3146–3154 (2009).
Tetsutani, K., To, H., Torii, M., Hisaeda, H. & Himeno, K. Malaria parasite induces tryptophan-related immune suppression in mice. Parasitology 134, 923–930 (2007).
Miu, J., Ball, H.J., Mellor, A.L. & Hunt, N.H. Effect of indoleamine dioxygenase-1 deficiency and kynurenine pathway inhibition on murine cerebral malaria. Int. J. Parasitol. 39, 363–370 (2009).
Wiesel, P. et al. Exacerbation of chronic renovascular hypertension and acute renal failure in heme oxygenase-1–deficient mice. Circ. Res. 88, 1088–1094 (2001).
Luria, A. et al. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J. Biol. Chem. 282, 2891–2898 (2007).
Wiesel, P. et al. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1–deficient mice. Circulation 102, 3015–3022 (2000).
Gramaglia, I. et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat. Med. 12, 1417–1422 (2006).
Mellor, A.L. et al. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J. Immunol. 171, 1652–1655 (2003).
Krege, J.H., Hodgin, J.B., Hagaman, J.R. & Smithies, O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25, 1111–1115 (1995).
Lau, A.K. et al. Probucol promotes functional reendothelialization in balloon-injured rabbit aortas. Circulation 107, 2031–2036 (2003).
Thomas, S.R., Chen, K. & Keaney, J.F., Jr. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase–dependent signaling pathway. J. Biol. Chem. 277, 6017–6024 (2002).
Palmer, D., Tsoi, K. & Maurice, D.H. Synergistic inhibition of vascular smooth muscle cell migration by phosphodiesterase 3 and phosphodiesterase 4 inhibitors. Circ. Res. 82, 852–861 (1998).
Becker, E.M. et al. The vasodilator-stimulated phosphoprotein (VASP): target of YC-1 and nitric oxide effects in human and rat platelets. J. Cardiovasc. Pharmacol. 35, 390–397 (2000).
Thomas, S.R., Mohr, D. & Stocker, R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferonγ-primed mononuclear phagocytes. J. Biol. Chem. 269, 14457–14464 (1994).
Christen, S., Peterhans, E. & Stocker, R. Antioxidant activities of some tryptophan metabolites: Possible implication for inflammatory diseases. Proc. Natl. Acad. Sci. USA 87, 2506–2510 (1990).
Hoenicka, M. et al. Purified soluble guanylyl cyclase expressed in a baculovirus/Sf9 system: stimulation by YC-1, nitric oxide and carbon monoxide. J. Mol. Med. 77, 14–23 (1999).
Schmidt, P., Schramm, M., Schröder, H. & Stasch, J.P. Preparation of heme-free soluble guanylate cyclase. Protein Expr. Purif. 31, 42–46 (2003).
Crawford, A., Hunt, N.H., Dawborn, J.K., Michelangeli, V.P. & Martin, T.J. Membranes from a transplantable osteogenic sarcoma responsive to parathyroid hormone and prostaglandins: regulation of adenylate cyclase and of hormone metabolism. J. Endocrinol. 77, 213–224 (1978).
Thomas, S.R. et al. Antioxidants inhibit indoleamine 2,3-dioxygenase in IFN-γ–activated human macrophages: posttranslational regulation by pyrrolidine dithiocarbamate. J. Immunol. 166, 6332–6340 (2001).
Acknowledgements
We thank E. Andriambeloson, K. Choy, M. Finnemore, A. Mitchell and H. Salahifar for assistance and preliminary experiments, J. Whitworth for the suggestion to test the effect of kynurenine on blood pressure in spontaneously hypertensive rats, G. Head for advice on telemetry and B.H. Chong (University of New South Wales) for providing MEG-01 cells. This work was supported by project grants G03S1177 from the Australian National Heart Foundation to R.S., 400992 and 401106 from the National Health & Medical Research Council (NHMRC) of Australia to R.S., DP0987074 from the Australian Research Council to N.H.H. 512469 from the NHMRC to N.H.H. and National Heart Foundation (OS 98S0008) and Australian Research Council (DP0343325) fellowships to P.K.W. R.S. holds an NHMRC Senior Principal Research Fellowship and University of Sydney Medical Foundation and Professorial Research Fellowships.
Author information
Authors and Affiliations
Contributions
J.F.K. Jr. and R.S. conceived of the project and performed initial experiments. Y.W., H.L., P.K.W., J.-P.S., D.C., B.J.W., S.R.T., D.S.C., J.F.K. Jr., N.H.H. and R.S. designed experiments and Y.W. performed most of the experiments and analyzed data. H.L. performed most experiments with porcine coronary arteries, G.M. performed immunohistochemistry, P.K.W. and S.R.T. performed experiments with endothelial cells, J.P.S. and M.H. performed experiments with isolated sGC, D.C. performed mouse endotoxemia experiments, B.J.W. performed experiments in spontaneous hypertensive rats, H.J.B. performed malarial infections of mice, V.K. established blood pressure measurements in unconscious rats and mice, and A.L.M generated Ido1-knockout mice. Y.W. and R.S. wrote and prepared the manuscript, with substantial contributions from H.L., J.F.K. Jr. and N.H.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1–16 and Supplementary Methods (PDF 1371 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, H., McKenzie, G. et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med 16, 279–285 (2010). https://doi.org/10.1038/nm.2092
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2092
This article is cited by
-
Yin/Yang associated differential responses to Psoralea corylifolia Linn. In rat models: an integrated metabolomics and transcriptomics study
Chinese Medicine (2023)
-
Spatial Metabolomics Reveals the Multifaceted Nature of Lamprey Buccal Gland and Its Diverse Mechanisms for Blood-Feeding
Communications Biology (2023)
-
Tryptophan challenge in individuals with schizophrenia and healthy controls: acute effects on circulating kynurenine and kynurenic acid, cognition and cerebral blood flow
Neuropsychopharmacology (2023)
-
Sexual Dimorphic Interplays Between Gut Microbiota and Antihypertensive Drugs
Current Hypertension Reports (2023)
-
Aryl hydrocarbon receptor–kynurenine axis promotes oncogenic activity in BCP-ALL
Cell Biology and Toxicology (2023)