Abstract
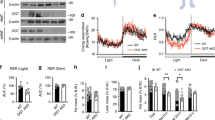
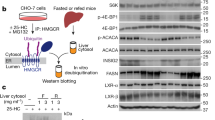
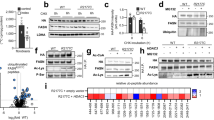
Animals are remarkably efficient in absorbing dietary fat and assimilating this energy-dense nutrient into the white adipose tissue (WAT) for storage. Although this metabolic efficiency may confer an advantage in times of calorie deprivation, it contributes to obesity and associated metabolic disorders when dietary fat is abundant1,2. Here we show that the intestinal lipid synthesis enzyme acyl CoA:monoacylglycerol acyltransferase-2 (MGAT2) has a crucial role in the assimilation of dietary fat and the accretion of body fat in mice. Mice lacking MGAT2 have a normal phenotype on a low-fat diet. However, on a high-fat diet, MGAT2-deficient mice are protected against developing obesity, glucose intolerance, hypercholesterolemia and fatty livers. Caloric intake is normal in MGAT2-deficient mice, and dietary fat is absorbed fully. However, entry of dietary fat into the circulation occurs at a reduced rate. This altered kinetics of fat absorption apparently results in more partitioning of dietary fat toward energy dissipation rather than toward storage in the WAT. Thus, our studies identify MGAT2 as a key determinant of energy metabolism in response to dietary fat and suggest that the inhibition of this enzyme may prove to be a useful strategy for treating obesity and other metabolic diseases associated with excessive fat intake.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Neel, J.V. Diabetes mellitus: a 'thrifty' genotype rendered detrimental by 'progress'? Am. J. Hum. Genet. 14, 353–362 (1962).
Muoio, D.M. & Newgard, C.B. Obesity-related derangements in metabolic regulation. Annu. Rev. Biochem. 75, 367–401 (2006).
The Food and Agricultural Organization of the United Nations. Food Consumption: Dietary Energy, Protein and Fat. http://www.fao.org/ faostat/foodsecurity/index_en.htm (2008).
Bell, R.M. & Coleman, R.A. Enzymes of glycerolipid synthesis in eukaryotes. Annu. Rev. Biochem. 49, 459–487 (1980).
Senior, J.R. & Isselbacher, K.J. Direct esterification of monoglycerides with palmityl coenzyme A by intestinal epithelial subcellular fractions. J. Biol. Chem. 237, 1454–1459 (1962).
Kayden, H.J., Senior, J.R. & Mattson, F.H. The monoglyceride pathway of fat absorption in man. J. Clin. Invest. 46, 1695–1703 (1967).
Mattson, F.H. & Volpenhein, R.A. The digestion and absorption of triglycerides. J. Biol. Chem. 239, 2772–2777 (1964).
Yen, C.-L.E., Stone, S.J., Cases, S., Zhou, P. & Farese, R.V., Jr. Identification of a gene encoding MGAT1, a monoacylglylcerol acyltransferase. Proc. Natl. Acad. Sci. USA 99, 8512–8517 (2002).
Yen, C.-L.E. & Farese, R.V., Jr. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 278, 18532–18537 (2003).
Cao, J., Lockwood, J., Burn, P. & Shi, Y. Cloning and functional characterization of a mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J. Biol. Chem. 278, 13860–13866 (2003).
Cheng, D. et al. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J. Biol. Chem. 278, 13611–13614 (2003).
Cao, J. et al. A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J. Biol. Chem. 279, 18878–18886 (2004).
Jandacek, R.J., Heubi, J.E. & Tso, P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology 127, 139–144 (2004).
Yen, C.-L.E., Monetti, M., Burri, B.J. & Farese, R.V., Jr. The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J. Lipid Res. 46, 1502–1511 (2005).
Baggio, L.L. & Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157 (2007).
Lowell, B.B. & Spiegelman, B.M. Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 (2000).
Krauss, S., Zhang, C.Y. & Lowell, B.B. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 6, 248–261 (2005).
Scrocchi, L.A. et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med. 2, 1254–1258 (1996).
Bouchard, C., Pérusse, L., Chagnon, Y., Warden, C. & Ricquier, D. Linkage between markers in the vicinity of the uncoupling protein 2 gene and resting metabolic rate in humans. Hum. Mol. Genet. 6, 1887–1889 (1997).
Snyder, F. & Stephens, N. A simplified spectrophotometric determination of ester groups in lipids. Biochim. Biophys. Acta 34, 244–245 (1959).
Hamilton, R.L., Jr., Goerke, J., Guo, L.S.S., Williams, M.C. & Havel, R.J. Unilamellar liposomes made with the French pressure cell: A simple preparative and semiquantitative technique. J. Lipid Res. 21, 981–992 (1980).
Iqbal, J. & Hussain, M. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J. Lipid Res. 46, 1491–1501 (2005).
Cases, S. et al. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276, 38870–38876 (2001).
Acknowledgements
We thank R. Jandacek at the University of Cincinnati Mouse Phenotyping Center for measuring lipid absorption; J.D. Fish for histological assistance; D. Walker for assistance with in situ hybridizations; D. Dubiel and K. Veenstra for measuring PYY and GLP-1; R. Bituin for mouse husbandry; Q. Walker for blastocyst microinjections; S. Ordway and G. Howard for editorial assistance; D. Jones for manuscript preparation; members of the Farese laboratory, M. Hirschey and M. Brown for insightful discussions; and R. Mahley and D. Srivastava for comments on the manuscript. This work was supported by funding from the American Heart Association (Scientist Development Grant to C.-L.E.Y.), the US National Institutes of Health (DK-056084 to R.V.F. Jr.), the US National Center for Research Resources (C06 RR018928), and the J. David Gladstone Institutes.
Author information
Authors and Affiliations
Contributions
C.-L.E.Y. designed and conducted experiments, coordinated the project and co-wrote the manuscript; M.-L.C., J.M., C.G., P.Z. and J.S.W. performed phenotyping experiments; B.H. and S.M. performed hormone assays; R.V.F. Jr directed the project and co-wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–5 and Suppmentary Methods (PDF 14912 kb)
Rights and permissions
About this article
Cite this article
Yen, CL., Cheong, ML., Grueter, C. et al. Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat Med 15, 442–446 (2009). https://doi.org/10.1038/nm.1937
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1937
This article is cited by
-
The monoacylglycerol acyltransferase pathway contributes to triacylglycerol synthesis in HepG2 cells
Scientific Reports (2022)
-
Methionine adenosyltransferase 1a antisense oligonucleotides activate the liver-brown adipose tissue axis preventing obesity and associated hepatosteatosis
Nature Communications (2022)
-
Engineering probiotics as living diagnostics and therapeutics for improving human health
Microbial Cell Factories (2020)
-
Metabolomics combined with network pharmacology exploration reveals the modulatory properties of Astragali Radix extract in the treatment of liver fibrosis
Chinese Medicine (2019)
-
A Review of the Evidence Supporting the Taste of Non‐esterified Fatty Acids in Humans
Journal of the American Oil Chemists' Society (2016)