Abstract
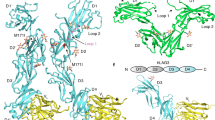
T cell receptor (TCR) binding degeneracy lies at the heart of several physiological and pathological phenomena, yet its structural basis is poorly understood. We determined the crystal structure of a complex involving the BM3.3 TCR and an octapeptide (VSV8) bound to the H-2Kb major histocompatibility complex molecule at a 2.7 Å resolution, and compared it with the BM3.3 TCR bound to the H-2Kb molecule loaded with a peptide that has no primary sequence identity with VSV8. Comparison of these structures showed that the BM3.3 TCR complementarity-determining region (CDR) 3α could undergo rearrangements to adapt to structurally different peptide residues. Therefore, CDR3 loop flexibility helps explain TCR binding cross-reactivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Davis, M.M. & Bjorkman, P.J. T-cell antigen receptor genes and T-cell recognition. Nature 334, 395–402 (1988).
Arstila, T.P. et al. A direct estimate of the human αβ T cell receptor diversity. Science 286, 958–961 (1999).
Mason, D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today 19, 395–404 (1998).
Bongrand, P. & Malissen, B. Quantitative aspects of T-cell recognition: from within the antigen-presenting cell to within the T cell. Bioessays 20, 412–422 (1998).
Eisen, H.N. Specificity and degeneracy in antigen recognition: yin and yang in the immune system. Annu. Rev. Immunol. 19, 1–21 (2001).
Langman, R.E. The specificity of immunological reactions. Mol. Immunol. 37, 555–561 (2000).
Goldrath, A.W. & Bevan, M.J. Selecting and maintaining a diverse T-cell repertoire. Nature 402, 255–262 (1999).
Benoist, C. & Mathis, D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat. Immunol. 2, 797–801 (2001).
Brehm, M.A. et al. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat. Immunol. 3, 627–634 (2002).
Wucherpfennig, K.W. & Strominger, J.L. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80, 695–705 (1995).
Evavold, B.D., Sloan-Lancaster, J., Wilson, K.J., Rothbard, J.B. & Allen, P.M. Specific T cell recognition of minimally homologous peptides: evidence for multiple endogenous ligands. Immunity 2, 655–663 (1995).
Lang, H.L. et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat. Immunol. 3, 940–943 (2002).
Luz, J.G. et al. Structural comparison of allogeneic and syngeneic T cell receptor-peptide-major histocompatibility complex complexes: a buried alloreactive mutation subtly alters peptide presentation substantially increasing V(β) interactions. J. Exp. Med. 195, 1175–1186 (2002).
Hennecke, J. & Wiley, D.C. Structure of a complex of the human α/β T cell receptor (TCR) HA1.7, influenza hemagglutinin peptide, and major histocompatibility complex class II molecule, HLA-DR4 (DRA*0101 and DRB1*0401): insight into TCR cross-restriction and alloreactivity. J. Exp. Med. 195, 571–581 (2002).
Ding, Y.H., Baker, B.M., Garboczi, D.N., Biddison, W.E. & Wiley, D.C. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity 11, 45–56 (1999).
Baker, B.M., Gagnon, S.J., Biddison, W.E. & Wiley, D.C. Conversion of a T cell antagonist into an agonist by repairing a defect in the TCR/peptide/MHC interface: implications for TCR signaling. Immunity 13, 475–484 (2000).
Rudolph, M.G. & Wilson, I.A. The specificity of TCR/pMHC interaction. Curr. Opin. Immunol. 14, 52–65 (2002).
Guimezanes, A. et al. Identification of endogenous peptides recognized by in vivo or in vitro generated alloreactive cytotoxic T lymphocytes: distinct characteristics correlated with CD8 dependence. Eur. J. Immunol. 31, 421–432 (2001).
Guimezanes, A., Schumacher, T.N., Ploegh, H.L. & Schmitt-Verhulst, A.M. A viral peptide can mimic an endogenous peptide for allorecognition of a major histocompatibility complex class I product. Eur. J. Immunol. 22, 1651–1654 (1992).
Reiser, J.-B. et al. Crystal structure of a T cell receptor bound to an allogeneic MHC molecule. Nat. Immunol. 1, 291–297 (2000).
Fremont, D.H., Matsumura, M., Stura, E.A., Peterson, P.A. & Wilson, I.A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science 257, 919–927 (1992).
Zhang, W., Young, A.C., Imarai, M., Nathenson, S.G. & Sacchettini, J.C. Crystal structure of the major histocompatibility complex class I H-2Kb molecule containing a single viral peptide: implications for peptide binding and T-cell receptor recognition. Proc. Natl. Acad. Sci. USA 89, 8403–8407 (1992).
Wu, L.C., Tuot, D.S., Lyons, D.S., Garcia, K.C. & Davis, M.M. Two-step binding mechanism for T-cell receptor recognition of peptide MHC. Nature 418, 552–556 (2002).
Garboczi, D.N. et al. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature 384, 134–141 (1996).
Garcia, K.C. et al. An αβ T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science 274, 209–219 (1996).
Lee, P.U., Churchill, H.R., Daniels, M., Jameson, S.C. & Kranz, D.M. Role of 2CT cell receptor residues in the binding of self- and allo-major histocompatibility complexes. J. Exp. Med. 191, 1355–1364 (2000).
Madden, D.R. The three-dimensional structure of peptide-MHC complexes. Annu. Rev. Immunol. 13, 587–622 (1995).
Degano, M. et al. A functional hot spot for antigen recognition in a superagonist TCR/MHC complex. Immunity 12, 251–261 (2000).
Ding, Y.H. et al. Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity 8, 403–411 (1998).
Reinherz, E.L. et al. The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science 286, 1913–1921 (1999).
Hennecke, J., Carfi, A. & Wiley, D.C. Structure of a covalently stabilized complex of a human αβ T-cell receptor, influenza HA peptide and MHC class II molecule, HLA-DR1. EMBO J. 19, 5611–5624 (2000).
Reiser, J.B. et al. A T cell receptor CDR3β loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity 16, 345–354 (2002).
Guimezanes, A., Montero-Julian, F. & Schmitt-Verhulst, A.-M. Unique structural features of a strong agonist permit co-receptor independence of an alloreactive TCR.
Kersh, G.J. et al. Structural and functional consequences of altering a peptide MHC anchor residue. J. Immunol. 166, 3345–3354 (2001).
Smith, K.J., Pyrdol, J., Gauthier, L., Wiley, D.C. & Wucherpfennig, K.W. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J. Exp. Med. 188, 1511–1520 (1998).
Wells, J.A. & de Vos, A.M. Hematopoietic receptor complexes. Annu. Rev. Biochem. 65, 609–634 (1996).
Radaev, S., Rostro, B., Brooks, A.G., Colonna, M. & Sun, P.D. Conformational plasticity revealed by the cocrystal structure of NKG2D and its class I MHC-like ligand ULBP3. Immunity 15, 1039–1049 (2001).
Keitel, T. et al. Crystallographic analysis of anti-p24 (HIV-1) monoclonal antibody cross-reactivity and polyspecificity. Cell 91, 811–820 (1997).
Wedemayer, G.J., Patten, P.A., Wang, L.H., Schultz, P.G. & Stevens, R.C. Structural insights into the evolution of an antibody combining site. Science 276, 1665–1669 (1997).
Garcia, K.C. et al. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science 279, 1166–1172 (1998).
Stanislawski, T. et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat. Immunol. 2, 962–970 (2001).
Grégoire, C., Malissen, B. & Mazza, G. Characterization of T cell receptor single-chain Fv fragments secreted by myeloma cells. Eur. J. Immunol. 26, 2410–2416 (1996).
O'Callaghan, C.A. et al. BirA enzyme: production and application in the study of membrane receptor-ligand interactions by site-specific biotinylation. Anal. Biochem. 266, 9–15 (1999).
Wyer, J.R. et al. T cell receptor and coreceptor CD8 αα bind peptide-MHC independently and with distinct kinetics. Immunity 10, 219–225 (1999).
Wlodawer, A. & Hodgson, K.O. Crystallisation and crystal data of monellin. Proc. Natl. Acad. Sci. USA 72, 398–399 (1975).
Leslie, A.G.W. in Crystallographic Computing (eds. Moras, D., Podjany, A.D. & Thierry, J.C.) 50–60 (Oxford University Press, Oxford, 1991).
Collaborative Computational Project Number 4. The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Hubbard, S.J. & Thornton, J.P. “NACCESS” Computer program (Department of Biochemistry and Molecular Biology, University College London, London, UK, 1993).
Satow, Y., Cohen, G.H., Padlan, E.A. & Davies, D.R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J. Mol. Biol. 190, 593–604 (1986).
Nicholls, A., Sharp, K.A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, 281–296 (1991).
Kraulis, P.J. Molscript: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Merrit, E.A. & Bacon, D.J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997).
Acknowledgements
We thank A. Guimezanes, A.-M. Schmitt-Verhulst, C. Kellenberger, L. Leserman and P. Golstein for discussions and comments on the manuscript, and C. Rieckel (beamline ID13), J.L. Ferrer (beamline FIP-BM30A), S. Arzt (beamline ID14-eh1), W. Burmeister (beamline ID14-eh3) and J. Lescar (beamline ID14-eh4) for help with synchrotron data collections at the ESRF (Grenoble). This work was supported by institutional grants from CNRS, CEA and INSERM, and specific grants from ARC, CNRS (programme PCV) and the European Communities (project EPI-PEP-VAC QLK2-CT-2002-00620). P.A.v.d.M. and A.K. are supported by the UK Medical Research Council.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Reiser, JB., Darnault, C., Grégoire, C. et al. CDR3 loop flexibility contributes to the degeneracy of TCR recognition. Nat Immunol 4, 241–247 (2003). https://doi.org/10.1038/ni891
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni891
This article is cited by
-
Structural understanding of T cell receptor triggering
Cellular & Molecular Immunology (2020)
-
DynaDom: structure-based prediction of T cell receptor inter-domain and T cell receptor-peptide-MHC (class I) association angles
BMC Structural Biology (2018)
-
Surface mediated cooperative interactions of drugs enhance mechanical forces for antibiotic action
Scientific Reports (2017)
-
The CD8 T‐cell response during tolerance induction in liver transplantation
Clinical & Translational Immunology (2016)
-
Direct molecular mimicry enables off-target cardiovascular toxicity by an enhanced affinity TCR designed for cancer immunotherapy
Scientific Reports (2016)