Abstract
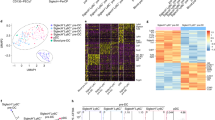
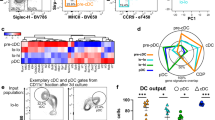
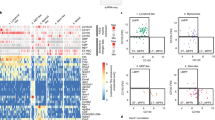
Lymphoid tissue plasmacytoid and conventional dendritic cells (DCs) are continuously regenerated from hematopoietic stem cells. The cytokine dependence and biology of plasmacytoid and conventional DCs suggest that regeneration might proceed through common DC-restricted developmental intermediates. By selecting for cytokine receptor expression relevant to DC development, we identify here highly cycling Lin−c-KitintFlt3+M-CSFR+ cells with a distinct gene-expression profile in mouse bone marrow that, on a clonal level in vitro and as a population both in vitro and in vivo, efficiently generated plasmacytoid and conventional DCs but no other lineages, which increased in number after in vivo injection of the cytokine Flt3 ligand. These clonogenic common DC progenitors thus define a cytokine-regulated DC developmental pathway that ensures the supply of various DC populations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Steinman, R.M., Hawiger, D. & Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Shortman, K. & Liu, Y.J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2, 151–161 (2002).
Kamath, A.T. et al. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J. Immunol. 165, 6762–6770 (2000).
Kabashima, K. et al. Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22, 439–450 (2005).
Liu, K. et al. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 8, 578–583 (2007).
Manz, M.G., Traver, D., Miyamoto, T., Weissman, I.L. & Akashi, K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood 97, 3333–3341 (2001).
Traver, D. et al. Development of CD8α+ dendritic cells from a common myeloid progenitor. Science 290, 2152–2154 (2000).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Kondo, M. et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21, 759–806 (2003).
Kondo, M., Weissman, I.L. & Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672 (1997).
Galy, A., Travis, M., Cen, D. & Chen, B. Human, T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity 3, 459–473 (1995).
Manz, M.G., Miyamoto, T., Akashi, K. & Weissman, I.L. Prospective isolation of human clonogenic common myeloid progenitors. Proc. Natl. Acad. Sci. USA 99, 11872–11877 (2002).
Chicha, L., Jarrossay, D. & Manz, M.G. Clonal type I interferon-producing and dendritic cell precursors are contained in both human lymphoid and myeloid progenitor populations. J. Exp. Med. 200, 1519–1524 (2004).
Shigematsu, H. et al. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity 21, 43–53 (2004).
D'Amico, A. & Wu, L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J. Exp. Med. 198, 293–303 (2003).
Karsunky, H., Merad, M., Cozzio, A., Weissman, I.L. & Manz, M.G. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 198, 305–313 (2003).
Mende, I., Karsunky, H., Weissman, I.L., Engleman, E.G. & Merad, M. Flk2+ myeloid progenitors are the main source of Langerhans cells. Blood 107, 1383–1390 (2006).
Lyman, S.D. & Jacobsen, S.E. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 91, 1101–1134 (1998).
Gilliet, M. et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195, 953–958 (2002).
McKenna, H.J. et al. Mice lacking Flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 95, 3489–3497 (2000).
Laouar, Y., Welte, T., Fu, X.Y. & Flavell, R.A. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity 19, 903–912 (2003).
Tussiwand, R., Onai, N., Mazzucchelli, L. & Manz, M.G. Inhibition of natural type I IFN-producing and dendritic cell development by a small molecule receptor tyrosine kinase inhibitor with Flt3 affinity. J. Immunol. 175, 3674–3680 (2005).
Maraskovsky, E. et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184, 1953–1962 (1996).
Miller, G., Pillarisetty, V.G., Shah, A.B., Lahrs, S. & DeMatteo, R.P. Murine Flt3 ligand expands distinct dendritic cells with both tolerogenic and immunogenic properties. J. Immunol. 170, 3554–3564 (2003).
Onai, N., Obata-Onai, A., Tussiwand, R., Lanzavecchia, A. & Manz, M.G. Activation of the Flt3 signal transduction cascade rescues and enhances type I interferon-producing and dendritic cell development. J. Exp. Med. 203, 227–238 (2006).
Caux, C. et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNFα. J. Exp. Med. 184, 695–706 (1996).
Inaba, K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 (1992).
Sallusto, F. & Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179, 1109–1118 (1994).
Vremec, D. et al. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur. J. Immunol. 27, 40–44 (1997).
Witmer-Pack, M.D. et al. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J. Cell. Sci. 104, 1021–1029 (1993).
Dai, X.M. et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111–120 (2002).
Ginhoux, F. et al. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7, 265–273 (2006).
MacDonald, K.P. et al. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J. Immunol. 175, 1399–1405 (2005).
Olweus, J. et al. Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin. Proc. Natl. Acad. Sci. USA 94, 12551–12556 (1997).
Bruno, L., Seidl, T. & Lanzavecchia, A. Mouse pre-immunocytes as non-proliferating multipotent precursors of macrophages, interferon-producing cells, CD8α+ and CD8α− dendritic cells. Eur. J. Immunol. 31, 3403–3412 (2001).
del Hoyo, G.M. et al. Characterization of a common precursor population for dendritic cells. Nature 415, 1043–1047 (2002).
Fogg, D.K. et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311, 83–87 (2006).
Naik, S.H. et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 7, 663–671 (2006).
Christensen, J.L. & Weissman, I.L. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. USA 98, 14541–14546 (2001).
Adolfsson, J. et al. Upregulation of Flt3 expression within the bone marrow Lin−Sca1+c-Kit+ stem cell compartment is accompanied by loss of self-renewal capacity. Immunity 15, 659–669 (2001).
Chklovskaia, E. et al. Reconstitution of dendritic and natural killer-cell subsets after allogeneic stem cell transplantation: effects of endogenous Flt3 ligand. Blood 103, 3860–3868 (2004).
Schotte, R., Nagasawa, M., Weijer, K., Spits, H. & Blom, B. The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development. J. Exp. Med. 200, 1503–1509 (2004).
Zenke, M. & Hieronymus, T. Towards an understanding of the transcription factor network of dendritic cell development. Trends Immunol. 27, 140–145 (2006).
Kanno, Y., Levi, B.Z., Tamura, T. & Ozato, K. Immune cell-specific amplification of interferon signaling by the IRF-4/8-PU.1 complex. J. Interferon Cytokine Res. 25, 770–779 (2005).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Cook, D.N. et al. Generation and analysis of mice lacking the chemokine fractalkine. Mol. Cell. Biol. 21, 3159–3165 (2001).
Wallny, H.J., Sollami, G. & Karjalainen, K. Soluble mouse major histocompatibility complex class II molecules produced in Drosophila cells. Eur. J. Immunol. 25, 1262–1266 (1995).
Marie, I., Durbin, J.E. & Levy, D.E. Differential viral induction of distinct interferon-α genes by positive feedback through interferon regulatory factor-7. EMBO J. 17, 6660–6669 (1998).
Acknowledgements
Supported by the Swiss National Science Foundation (310000-116637), the Deutsche Forschungsgemeinschaft (MA 2159/2-1) and the European Commission FP6 Network of Excellence initiative (LSHB-CT-2004-512074 DC-THERA).
Author information
Authors and Affiliations
Contributions
N.O., A.O.-O. and M.A.S. designed experiments, did experiments, collected data and contributed to the writing of the manuscript; T.O. provided advice; D.J. did cell sorting; and M.G.M. directed the study and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 548 kb)
Rights and permissions
About this article
Cite this article
Onai, N., Obata-Onai, A., Schmid, M. et al. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol 8, 1207–1216 (2007). https://doi.org/10.1038/ni1518
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1518
This article is cited by
-
Transitional dendritic cells are distinct from conventional DC2 precursors and mediate proinflammatory antiviral responses
Nature Immunology (2023)
-
Skull bone marrow channels as immune gateways to the central nervous system
Nature Neuroscience (2023)
-
Traumatic brain injury alters dendritic cell differentiation and distribution in lymphoid and non-lymphoid organs
Journal of Neuroinflammation (2022)
-
Endothelial cell-specific expression of serine/threonine kinase 11 modulates dendritic cell differentiation
Nature Communications (2022)
-
Ly6D+Siglec-H+ precursors contribute to conventional dendritic cells via a Zbtb46+Ly6D+ intermediary stage
Nature Communications (2022)