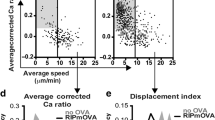
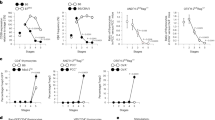
Abstract
Thymocytes displaying self-reactive T cell receptors usually undergo negative selection in the thymus. Here we demonstrate that agonist peptides can promote positive selection of immature double-positive thymocytes into distinct lineages, varying with the agonist concentration and the animal's age. Microarray gene expression analyses showed broad transcriptional alterations in a set of transcripts associated with the innate immune system, as well as silencing of CD8β expression. The resulting CD8αα T cells showed a rapid effector cytokine response. Hence, T cells displaying self-reactive receptors can have the gene expression profile and phenotypic characteristics of innate immune cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Germain, R.N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2, 309–322 (2002).
Sebzda, E. et al. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science 263, 1615–1618 (1994).
Ashton-Rickardt, P.G. et al. Evidence for a differential avidity model of T cell selection in the thymus. Cell 76, 651–663 (1994).
Hogquist, K.A., Jameson, S.C. & Bevan, M.J. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+ T cells. Immunity 3, 79–86 (1995).
Kawai, K. & Ohashi, P.S. Immunological function of a defined T-cell population tolerized to low-affinity self antigens. Nature 374, 68–69 (1995).
Girao, C., Hu, Q., Sun, J. & Ashton-Rickardt, P.G. Limits to the differential avidity model of T cell selection in the thymus. J. Immunol. 159, 4205–4211 (1997).
Wang, R., Nelson, A., Kimachi, K., Grey, H.M. & Farr, A.G. The role of peptides in thymic positive selection of class II major histocompatibility complex-restricted T cells. Proc. Natl. Acad. Sci. USA 95, 3804–3809 (1998).
Kraj, P. et al. Positive selection of CD4+ T cells is induced in vivo by agonist and inhibited by antagonist peptides. J. Exp. Med. 194, 407–416 (2001).
Jordan, M.S. et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2, 283–284 (2001).
Apostolou, I., Sarukhan, A., Klein, L. & von Boehmer, H. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3, 756–763 (2002).
Park, S.H. et al. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J. Exp. Med. 193, 893–904 (2001).
Burnet, F.M. A modification of Jerne's theory of antibody production using the concept of clonal selection. C.A. Cancer J. Clin. 26, 119–121 (1976).
von Boehmer, H., Teh, H.S. & Kisielow, P. The thymus selects the useful, neglects the useless and destroys the harmful. Immunol. Today 10, 57–61 (1989).
Schwartz, R.H. in Fundamental Immunology (ed. Paul, W.E.) 701–739 (Lippincott-Raven, Philadelphia, 1999).
Anderson, M.S. et al. Projection of an immunological self-shadow within the thymus by the aire protein. Science 298, 139–1401 (2002).
Anderson, G., Jenkinson, E.J., Moore, N.C. & Owen, J.J. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature 362, 70–73 (1993).
Schulz, R.J., Parkes, A., Mizoguchi, E., Bhan, A.K. & Koyasu, S. Development of CD4−CD8− αβ TCR+NK1.1+ T lymphocytes: thymic selection by self antigen. J. Immunol. 157, 4379–4389 (1996).
Yasutomo, K., Doyle, C., Miele, L. & Germain, R.N. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature 404, 506–510 (2000).
Rocha, B., von Boehmer, H. & Guy-Grand, D. Selection of intraepithelial lymphocytes with CD8α/α co-receptors by self-antigen in the murine gut. Proc. Natl. Acad. Sci. USA 89, 5336–5340 (1992).
Cruz, D. et al. An opposite pattern of selection of a single T cell antigen receptor in the thymus and among intraepithelial lymphocytes. J. Exp. Med. 188, 255–265 (1998).
Leishman, A.J. et al. Precursors of functional MHC class I- or class II-restricted CD8αα+ T cells are positively selected in the thymus by agonist self-peptides. Immunity 16, 355–364 (2002).
Chidgey, A. & Boyd, R. Agonist peptide modulates T cell selection thresholds through qualitative and quantitative shifts in CD8 co-receptor expression. Int. Immunol. 9, 1527–1536 (1997).
Barnden, M.J., Heath, W.R. & Carbone, F.R. Down-modulation of CD8 β-chain in response to an altered peptide ligand enables developing thymocytes to escape negative selection. Cell. Immunol. 175, 111–119 (1997).
Hogquist, K.A. & Bonnevier, J.L. Development of peptide-selected CD8 T cells in fetal thymic organ culture occurs via the conventional pathway. J. Immunol. 161, 3896–3901 (1998).
Teh, H.S. et al. Early deletion and late positive selection of T cells expressing a male-specific receptor in T-cell receptor transgenic mice. Dev. Immunol. 1, 1–10 (1990).
Teh, H.S., Kishi, H., Scott, B. & von Boehmer, H. Deletion of autospecific T cells in T cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J. Exp. Med. 169, 795–806 (1989).
Kisielow, P., Bluthmann, H., Staerz, U.D., Steinmetz, M. & von Boehmer, H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 333, 742–746 (1988).
Berg, L.J., Frank, G.D. & Davis, M.M. The effects of MHC gene dosage and allelic variation on T cell receptor selection. Cell 60, 1043–1053 (1990).
Kersh, G.J., Engle, D.L., Williams, C.B. & Allen, P.M. Ligand-specific selection of MHC class II-restricted thymocytes in fetal thymic organ culture. J. Immunol. 164, 5675–5682 (2000).
Irizarry, R.A. et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15 (2003).
Moretta, A. et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19, 197–223 (2001).
Yokota, Y. et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397, 702–706 (1999).
Barton, K. et al. The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity 9, 555–563 (1998).
Luster, A.D. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 14, 129–135 (2002).
Vorbach, C., Harrison, R. & Capecchi, M.R. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 24, 512–517 (2003).
Hacker, C. et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat. Immunol. 4, 380–386 (2003).
Bendelac, A., Bonneville, M. & Kearney, J.F. Autoreactivity by design: innate B and T lymphocytes. Nat. Rev. Immunol. 1, 177–186 (2001).
Teh, H.S. et al. Thymic major histocompatibility complex antigens and the αβ T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature 335, 229–233 (1988).
Bruno, L., Fehling, H.J. & von Boehmer, H. The αβ T cell receptor can replace the γδ receptor in the development of γδ lineage cells. Immunity 5, 343–352 (1996).
Buch, T., Rieux-Laucat, F., Forster, I. & Rajewsky, K. Failure of HY-specific thymocytes to escape negative selection by receptor editing. Immunity 16, 707–718 (2002).
Gao, G.F., Rao, Z. & Bell, J.I. Molecular coordination of αβ T-cell receptors and coreceptors CD8 and CD4 in their recognition of peptide-MHC ligands. Trends Immunol. 23, 408–413 (2002).
Bain, G. et al. E2A deficiency leads to abnormalities in αβ T-cell development and to rapid development of T-cell lymphomas. Mol. Cell. Biol. 17, 4782–4791 (1997).
Capone, M. et al. Selective absence of CD8+ TCRαβ+ intestinal epithelial cells in transgenic mice expressing β2-microglobulin-associated ligands exclusively on thymic cortical epithelium. Eur. J. Immunol. 33, 1471–1477 (2003).
Gleimer, M. & Parham, P. Stress management: MHC class I and class I-like molecules as reporters of cellular stress. Immunity 19, 469–477 (2003).
Natarajan, K., Dimasi, N., Wang, J., Mariuzza, R.A. & Margulies, D.H. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu. Rev. Immunol. 20, 853–885 (2002).
McMahon, C.W. & Raulet, D.H. Expression and function of NK cell receptors in CD8+ T cells. Curr. Opin. Immunol. 13, 465–470 (2001).
Shires, J., Theodoridis, E. & Hayday, A.C. Biological insights into TCRγδ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity 15, 419–434 (2001).
Acknowledgements
We thank L. Poirot, E. Venanzi and A. Goldrath for microarray data sets; Q.-M. Pham and V. Bruklich for help with the mice; G. Losyev for flow cytometry; R. Park for help with the data analysis; and the T cell group for discussions. Supported by the National Institutes of Health (1R01AI51530), by core services from the National Institute of Diabetes and Digestive and Kidney Diseases–funded Diabetes Endocrinology Research Center (Joslin Diabetes Center) and by the Japan Society for the Promotion of Science and the Uehara Memorial Foundation (T.Y.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yamagata, T., Mathis, D. & Benoist, C. Self-reactivity in thymic double-positive cells commits cells to a CD8αα lineage with characteristics of innate immune cells. Nat Immunol 5, 597–605 (2004). https://doi.org/10.1038/ni1070
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1070
This article is cited by
-
The transcription factor LRF promotes integrin β7 expression by and gut homing of CD8αα+ intraepithelial lymphocyte precursors
Nature Immunology (2022)
-
The histone demethylase Kdm6b regulates the maturation and cytotoxicity of TCRαβ+CD8αα+ intestinal intraepithelial lymphocytes
Cell Death & Differentiation (2022)
-
Differential expression of tissue-restricted antigens among mTEC is associated with distinct autoreactive T cell fates
Nature Communications (2020)
-
Single-cell RNA-sequencing identifies the developmental trajectory of C-Myc-dependent NK1.1− T-bet+ intraepithelial lymphocyte precursors
Mucosal Immunology (2020)
-
Human intraepithelial lymphocytes
Mucosal Immunology (2018)