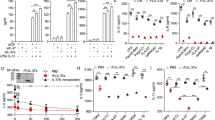
Abstract
Endotoxin tolerance, a key mechanism for suppressing excessive inflammatory cytokine production, is induced by prior exposure of macrophages to Toll-like receptor (TLR) ligands. Induction of cross-tolerance to endotoxin by endogenous cytokines has not been investigated. Here we show that prior exposure to tumor necrosis factor (TNF) induced a tolerant state in macrophages, with less cytokine production after challenge with lipopolysaccharide (LPS) and protection from LPS-induced death. TNF-induced cross-tolerization was mediated by suppression of LPS-induced signaling and chromatin remodeling. TNF-induced cross-tolerance was dependent on the kinase GSK3, which suppressed chromatin accessibility and promoted rapid termination of signaling via the transcription factor NF-κB by augmenting negative feedback by the signaling inhibitors A20 and IκBα. Our results demonstrate an unexpected homeostatic function for TNF and a GSK3-mediated mechanism for the prevention of prolonged and excessive inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Beutler, B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol. Rev. 227, 248–263 (2009).
Liew, F.Y., Xu, D., Brint, E.K. & O'Neill, L.A.J. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 (2005).
Biswas, S.K. & Lopez-Collazo, E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 (2009).
Foster, S.L., Hargreaves, D.C. & Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 (2007).
Foster, S.L. & Medzhitov, R. Gene-specific control of the TLR-induced inflammatory response. Clin. Immunol. 130, 7–15 (2009).
Smale, S.T. Selective transcription in response to an inflammatory stimulus. Cell 140, 833–844 (2010).
Nathan, C. & Ding, A. Nonresolving inflammation. Cell 140, 871–882 (2010).
Hodge-Dufour, J. et al. Inhibition of interferon γ induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 95, 13806–13811 (1998).
Williams-Skipp, C. et al. Unmasking of a protective tumor necrosis factor receptor I-mediated signal in the collagen-induced arthritis model. Arthritis Rheum. 60, 408–418 (2009).
Blüml, S. et al. Antiinflammatory effects of tumor necrosis factor on hematopoietic cells in a murine model of erosive arthritis. Arthritis Rheum. 62, 1608–1619 (2010).
Zakharova, M. & Ziegler, H.K. Paradoxical anti-inflammatory actions of TNF-α: inhibition of IL-12 and IL-23 via TNF receptor 1 in macrophages and dendritic cells. J. Immunol. 175, 5024–5033 (2005).
Marino, M.W. et al. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA 94, 8093–8098 (1997).
Kollias, G., Douni, E., Kassiotis, G. & Kontoyiannis, D. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol. Rev. 169, 175–194 (1999).
Banchereau, J. & Pascual, V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392 (2006).
Doble, B.W. & Woodgett, J.R. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 (2003).
Martin, M., Rehani, K., Jope, R.S. & Michalek, S.M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 6, 777–784 (2005).
Hu, X. et al. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 24, 563–574 (2006).
Beurel, E., Michalek, S.M. & Jope, R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 31, 24–31 (2010).
Steinbrecher, K.A., Wilson, W., Cogswell, P.C. & Baldwin, A.S. Glycogen synthase kinase 3β functions to specify gene-specific, NF-κB-dependent transcription. Mol. Cell. Biol. 25, 8444–8455 (2005).
Buss, H. et al. Phosphorylation of serine 468 by GSK-3β negatively regulates basal p65 NF-κB activity. J. Biol. Chem. 279, 49571–49574 (2004).
Saijo, K. et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137, 47–59 (2009).
Takada, Y. et al. Genetic deletion of glycogen synthase kinase-3β abrogates activation of IκBα kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J. Biol. Chem. 279, 39541–39554 (2004).
Hoeflich, K.P. et al. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 406, 86–90 (2000).
Rodionova, E. et al. GSK-3 mediates differentiation and activation of proinflammatory dendritic cells. Blood 109, 1584–1592 (2007).
Shen, F. et al. IL-17 receptor signaling inhibits C/EBPβ by sequential phosphorylation of the regulatory 2 domain. Sci. Signal. 2, ra8 (2009).
Shen, E., Fan, J. & Peng, T. Glycogen synthase kinase-3β suppresses tumor necrosis factor-α expression in cardiomyocytes during lipopolysaccharide stimulation. J. Cell. Biochem. 104, 329–338 (2008).
Vines, A. et al. Novel anti-inflammatory role for glycogen synthase kinase-3β in the inhibition of tumor necrosis factor-α- and interleukin-1β-induced inflammatory gene expression. J. Biol. Chem. 281, 16985–16990 (2006).
Jafferany, M. Lithium and skin: dermatologic manifestations of lithium therapy. Int. J. Dermatol. 47, 1101–1111 (2008).
del Fresno, C. et al. Inflammatory responses associated with acute coronary syndrome up-regulate IRAK-M and induce endotoxin tolerance in circulating monocytes. J. Endotoxin Res. 13, 39–52 (2007).
Kawasaki, T. et al. Surgical stress induces endotoxin hyporesponsiveness and an early decrease of monocyte mCD14 and HLA-DR expression during surgery. Anesth. Analg. 92, 1322–1326 (2001).
Langdale, L.A., Kajikawa, O., Frevert, C. & Liggitt, H.D. Sustained tolerance to lipopolysaccharide after liver ischemia-reperfusion injury. Shock 19, 553–558 (2003).
Chen, J. & Ivashkiv, L.B. IFN-γ abrogates endotoxin tolerance by facilitating Toll-like receptor-induced chromatin remodeling. Proc. Natl. Acad. Sci. USA 107, 19438–19443 (2010).
Hayden, M.S. & Ghosh, S. Shared principles in NF-κB signaling. Cell 132, 344–362 (2008).
Boone, D.L. et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060 (2004).
Patel, S. et al. Tissue-specific role of glycogen synthase kinase 3β in glucose homeostasis and insulin action. Mol. Cell. Biol. 28, 6314–6328 (2008).
Werner, S.L. et al. Encoding NF-κB temporal control in response to TNF: distinct roles for the negative regulators IκBα and A20. Genes Dev. 22, 2093–2101 (2008).
Saccani, S., Pantano, S. & Natoli, G. p38-Dependent marking of inflammatory genes for increased NF-κB recruitment. Nat. Immunol. 3, 69–75 (2002).
Weinmann, A.S. et al. Nucleosome remodeling at the IL-12 p40 promoter is a TLR-dependent, Rel-independent event. Nat. Immunol. 2, 51–57 (2001).
Ramirez-Carrozzi, V.R. et al. Selective and antagonistic functions of SWI/SNF and Mi-2β nucleosome remodeling complexes during an inflammatory response. Genes Dev. 20, 282–296 (2006).
Aggarwal, B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745–756 (2003).
Christen, U. et al. A dual role for TNF-α in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J. Immunol. 166, 7023–7032 (2001).
Hassett, S., Moynagh, P. & Reen, D. TNF-α is a mediator of the anti-inflammatory response in a human neonatal model of the non-septic shock syndrome. Pediatr. Surg. Int. 22, 24–30 (2006).
Kassiotis, G. & Kollias, G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J. Exp. Med. 193, 427–434 (2001).
Stahl, E.A. et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat. Genet. 42, 508–514 (2010).
Plenge, R.M. et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat. Genet. 39, 1477–1482 (2007).
Natoli, G., Saccani, S., Bosisio, D. & Marazzi, I. Interactions of NF-κB with chromatin: the art of being at the right place at the right time. Nat. Immunol. 6, 439–445 (2005).
Werner, S.L., Barken, D. & Hoffmann, A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science 309, 1857–1861 (2005).
Yarilina, A. et al. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat. Immunol. 9, 378–387 (2008).
Acknowledgements
We thank G.D. Kalliolias and A. Yarilina for discussions and J. Woodgett (University of Toronto) for mice with loxP-flanked alleles encoding GSK3β. Supported by the National Institutes of Health (L.B.I.).
Author information
Authors and Affiliations
Contributions
S.H.P. designed and did experiments and wrote the manuscript; K.-H.P.-M. contributed to the signaling experiments; J.C. contributed to the restriction-enzyme accessibility experiments; X.H. contributed to the in vivo experiments; and L.B.I. designed and supervised the research and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 2777 kb)
Rights and permissions
About this article
Cite this article
Park, S., Park-Min, KH., Chen, J. et al. Tumor necrosis factor induces GSK3 kinase–mediated cross-tolerance to endotoxin in macrophages. Nat Immunol 12, 607–615 (2011). https://doi.org/10.1038/ni.2043
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2043
This article is cited by
-
CytoSorb hemoperfusion markedly attenuates circulating cytokine concentrations during systemic inflammation in humans in vivo
Critical Care (2023)
-
The crucial regulatory role of type I interferon in inflammatory diseases
Cell & Bioscience (2023)
-
A20 promotes colorectal cancer immune evasion by upregulating STC1 expression to block “eat-me” signal
Signal Transduction and Targeted Therapy (2023)
-
Arsenic and Tau Phosphorylation: a Mechanistic Review
Biological Trace Element Research (2023)
-
Methacrylic Acid-Based Regenerative Biomaterials: Explorations into the MAAgic
Regenerative Engineering and Translational Medicine (2023)