Abstract
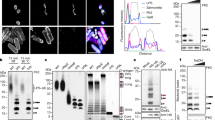
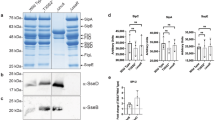
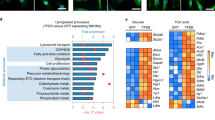
Cell-autonomous innate immune responses against bacteria attempting to colonize the cytosol of mammalian cells are incompletely understood. Polyubiquitylated proteins can accumulate on the surface of such bacteria, and bacterial growth is restricted by Tank-binding kinase (TBK1). Here we show that NDP52, not previously known to contribute to innate immunity, recognizes ubiquitin-coated Salmonella enterica in human cells and, by binding the adaptor proteins Nap1 and Sintbad, recruits TBK1. Knockdown of NDP52 and TBK1 facilitated bacterial proliferation and increased the number of cells containing ubiquitin-coated salmonella. NDP52 also recruited LC3, an autophagosomal marker, and knockdown of NDP52 impaired autophagy of salmonella. We conclude that human cells utilize the ubiquitin system and NDP52 to activate autophagy against bacteria attempting to colonize their cytosol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Coburn, B., Grassl, G.A. & Finlay, B.B. Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 85, 112–118 (2007).
Haraga, A., Ohlson, M.B. & Miller, S.I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6, 53–66 (2008).
McGhie, E.J., Brawn, L.C., Hume, P.J., Humphreys, D. & Koronakis, V. Salmonella takes control: effector-driven manipulation of the host. Curr. Opin. Microbiol. 12, 117–124 (2009).
Perrin, A.J., Jiang, X., Birmingham, C.L., So, N.S. & Brumell, J.H. Recognition of bacteria in the cytosol of mammalian cells by the ubiquitin system. Curr. Biol. 14, 806–811 (2004).
Radtke, A.L., Delbridge, L.M., Balachandran, S., Barber, G.N. & O'Riordan, M.X. TBK1 protects vacuolar integrity during intracellular bacterial infection. PLoS Pathog. 3, e29 (2007).
Radtke, A.L. & O'Riordan, M.X. Homeostatic maintenance of pathogen-containing vacuoles requires TBK1-dependent regulation of aquaporin-1. Cell. Microbiol. 10, 2197–2207 (2008).
Fitzgerald, K.A. et al. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
Hemmi, H. et al. The roles of two IκB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199, 1641–1650 (2004).
McWhirter, S.M. et al. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. USA 101, 233–238 (2004).
Ishii, K.J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729 (2008).
Wu, C.J., Conze, D.B., Li, T., Srinivasula, S.M. & Ashwell, J.D. NEMO is a sensor of Lys 63-linked polyubiquitination and functions in NF-κB activation. Nat. Cell Biol. 8, 398–406 (2006).
Ea, C.K., Deng, L., Xia, Z.P., Pineda, G. & Chen, Z.J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006).
Bloor, S. et al. Signal processing by its coil zipper domain activates IKK gamma. Proc. Natl. Acad. Sci. USA 105, 1279–1284 (2008).
Lo, Y.C. et al. Structural basis for recognition of diubiquitins by NEMO. Mol. Cell 33, 602–615 (2009).
Rahighi, S. et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136, 1098–1109 (2009).
Birmingham, C.L., Smith, A.C., Bakowski, M.A., Yoshimori, T. & Brumell, J.H. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281, 11374–11383 (2006).
Pomerantz, J.L. & Baltimore, D. NF-κB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 18, 6694–6704 (1999).
Fujita, F. et al. Identification of NAP1, a regulatory subunit of IκB kinase-related kinases that potentiates NF-κB signaling. Mol. Cell. Biol. 23, 7780–7793 (2003).
Bouwmeester, T. et al. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat. Cell Biol. 6, 97–105 (2004).
Ryzhakov, G. & Randow, F. SINTBAD, a novel component of innate antiviral immunity, shares a TBK1-binding domain with NAP1 and TANK. EMBO J. 26, 3180–3190 (2007).
Sasai, M. et al. Cutting Edge: NF-κB-activating kinase-associated protein 1 participates in TLR3/Toll-IL-1 homology domain-containing adapter molecule-1-mediated IFN regulatory factor 3 activation. J. Immunol. 174, 27–30 (2005).
Sasai, M. et al. NAK-associated protein 1 participates in both the TLR3 and the cytoplasmic pathways in type I IFN induction. J. Immunol. 177, 8676–8683 (2006).
Guo, B. & Cheng, G. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J. Biol. Chem. 282, 11817–11826 (2007).
Barrios-Rodiles, M. et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307, 1621–1625 (2005).
Zhao, T. et al. The NEMO adaptor bridges the nuclear factor-κB and interferon regulatory factor signaling pathways. Nat. Immunol. 8, 592–600 (2007).
Morton, S., Hesson, L., Peggie, M. & Cohen, P. Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS Lett. 582, 997–1002 (2008).
Korioth, F., Gieffers, C., Maul, G.G. & Frey, J. Molecular characterization of NDP52, a novel protein of the nuclear domain 10, which is redistributed upon virus infection and interferon treatment. J. Cell Biol. 130, 1–13 (1995).
Sternsdorf, T., Jensen, K., Zuchner, D. & Will, H. Cellular localization, expression, and structure of the nuclear dot protein 52. J. Cell Biol. 138, 435–448 (1997).
Morriswood, B. et al. T6BP and NDP52 are myosin VI binding partners with potential roles in cytokine signalling and cell adhesion. J. Cell Sci. 120, 2574–2585 (2007).
Gurung, R. et al. Identification of a novel domain in two mammalian inositol-polyphosphate 5-phosphatases that mediates membrane ruffle localization. The inositol 5-phosphatase skip localizes to the endoplasmic reticulum and translocates to membrane ruffles following epidermal growth factor stimulation. J. Biol. Chem. 278, 11376–11385 (2003).
Iha, H. et al. Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-κB activation. EMBO J. 27, 629–641 (2008).
Nakagawa, I. et al. Autophagy defends cells against invading group A Streptococcus. Science 306, 1037–1040 (2004).
Ray, K., Marteyn, B., Sansonetti, P.J. & Tang, C.M. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat. Rev. Microbiol. 7, 333–340 (2009).
Virgin, H.W. & Levine, B. Autophagy genes in immunity. Nat. Immunol. 10, 461–470 (2009).
Bielecki, J., Youngman, P., Connelly, P. & Portnoy, D.A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature 345, 175–176 (1990).
Goetz, M. et al. Microinjection and growth of bacteria in the cytosol of mammalian host cells. Proc. Natl. Acad. Sci. USA 98, 12221–12226 (2001).
Ogawa, M. et al. Escape of intracellular Shigella from autophagy. Science 307, 727–731 (2005).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008).
Yano, T. et al. Autophagic control of listeria through intracellular innate immune recognition in Drosophila. Nat. Immunol. 9, 908–916 (2008).
Hiemstra, P.S., van den Barselaar, M.T., Roest, M., Nibbering, P.H. & van Furth, R. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J. Leukoc. Biol. 66, 423–428 (1999).
Beuzon, C.R., Salcedo, S.P. & Holden, D.W. Growth and killing of a Salmonella enterica serovar Typhimurium sifA mutant strain in the cytosol of different host cell lines. Microbiology 148, 2705–2715 (2002).
Bjorkoy, G. et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614 (2005).
Komatsu, M. et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131, 1149–1163 (2007).
Pankiv, S. et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145 (2007).
Kirkin, V., Lamark, T., Johansen, T. & Dikic, I. NBR1 cooperates with p62 in selective autophagy of ubiquitinated targets. Autophagy 5, 732–733 (2009).
Puls, A., Schmidt, S., Grawe, F. & Stabel, S. Interaction of protein kinase C zeta with ZIP, a novel protein kinase C-binding protein. Proc. Natl. Acad. Sci. USA 94, 6191–6196 (1997).
Sanchez, P., De Carcer, G., Sandoval, I.V., Moscat, J. & Diaz-Meco, M.T. Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol. Cell. Biol. 18, 3069–3080 (1998).
Randow, F. & Sale, J.E. Retroviral transduction of DT40. Subcell. Biochem. 40, 383–386 (2006).
Randow, F. & Seed, B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 3, 891–896 (2001).
Krumbach, R., Bloor, S., Ryzhakov, G. & Randow, F. Somatic cell genetics for the study of NF-κB signaling in innate immunity. Science Signaling 1, part 7 2008.
Acknowledgements
We thank F. Begum and S.-Y. Peak-Chew for mass spectrometry, J. Kendrick-Jones (Medical Research Council Laboratory of Molecular Biology) for NDP52 antiserum, D. Holden (Imperial College London), C. Tang (Imperial College London), S. Sriskandan (Imperial College London) and C. Bryant (University of Cambridge) for bacterial strains and advice, A. Geerlof (European Molecular Biology Laboratory Heidelberg) for pETM plasmid, and D. Fearon, P. Lehner and D. Komander for comments.
Author information
Authors and Affiliations
Contributions
T.L.M.T., G.R., S.B. and N.v.M. performed experiments and analyzed data. F.R. designed the overall research and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Table 1 (PDF 2494 kb)
Rights and permissions
About this article
Cite this article
Thurston, T., Ryzhakov, G., Bloor, S. et al. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol 10, 1215–1221 (2009). https://doi.org/10.1038/ni.1800
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1800
This article is cited by
-
Optineurin provides a mitophagy contact site for TBK1 activation
The EMBO Journal (2024)
-
The role of NEDD4 related HECT-type E3 ubiquitin ligases in defective autophagy in cancer cells: molecular mechanisms and therapeutic perspectives
Molecular Medicine (2023)
-
ATG7 and ATG14 restrict cytosolic and phagosomal Mycobacterium tuberculosis replication in human macrophages
Nature Microbiology (2023)
-
SB2301-mediated perturbation of membrane composition in lipid droplets induces lipophagy and lipid droplets ubiquitination
Communications Biology (2023)
-
NDP52 mediates an antiviral response to hepatitis B virus infection through Rab9-dependent lysosomal degradation pathway
Nature Communications (2023)