Abstract
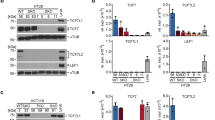
Constitutive activation of the Wnt signaling pathway is a root cause of many colon cancers1,2,3. Activation of this pathway is caused by genetic mutations that stabilize the β-catenin protein, allowing it to accumulate in the nucleus and form complexes with any member of the lymphoid enhancer factor (LEF1) and T-cell factor (TCF1, TCF3, TCF4) family of transcription factors (referred to collectively as LEF/TCFs) to activate transcription of target genes3,4. Target genes such as MYC, CCND1, MMP7 and TCF7 (refs. 5–9) are normally expressed in colon tissue, so it has been proposed that abnormal expression levels or patterns imposed by β-catenin/TCF complexes have a role in tumor progression. We report here that LEF1 is a new type of target gene ectopically activated in colon cancer. The pattern of this ectopic expression is unusual because it derives from selective activation of a promoter for a full-length LEF1 isoform that binds β-catenin, but not a second, intronic promoter that drives expression of a dominant-negative isoform. β-catenin/TCF complexes can activate the promoter for full-length LEF1, indicating that in cancer high levels of these complexes misregulate transcription to favor a positive feedback loop for Wnt signaling by inducing selective expression of full-length, β-catenin–sensitive forms of LEF/TCFs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kinzler, K. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Polakis, P. Wnt signaling and cancer. Genes Dev. 14, 1837–1851 (2000).
Roose, J. & Clevers, H. TCF transcription factors: molecular switches in carcinogenesis. Biochim. Biophys. Acta 1424, M23–37 (1999).
Polakis, P., Hart, M. & Rubinfeld, B. Defects in the regulation of β-catenin in colorectal cancer. Adv. Exp. Med. Biol. 470, 23–32 (1999).
Roose, J. et al. Synergy between tumor suppressor APC and the β-catenin-Tcf4 target Tcf1. Science 285, 1923–1926 (1999).
He, T.C. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998).
Tetsu, O. & McCormick, F. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–426 (1999).
Crawford, H.C. et al. The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 18, 2883–2891 (1999).
Brabletz, T., Jung, A., Dag, S., Hlubek, F. & Kirchner, T. β-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am. J. Pathol. 155, 1033–1038 (1999).
Porfiri, E. et al. Induction of a β-catenin-LEF-1 complex by wnt-1 and transforming mutants of β-catenin. Oncogene 15, 2833–2839 (1997).
Korinek, V. et al. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787 (1997).
Waterman, M.L., Fischer, W.H. & Jones, K.A. A thymus-specific member of the HMG protein family regulates the human T cell receptor C α enhancer. Genes Dev. 5, 656–669 (1991).
Travis, A., Amsterdam, A., Belanger, C. & Grosschedl, R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor α enhancer function. Genes Dev. 5, 880–894 (1991).
Hovanes, K., Li, T.W.H. & Waterman, M.L. The human LEF-1 gene contains a promoter preferentially active in lymphocytes and encodes multiple isoforms derived from alternative splicing. Nucleic Acids Res. 28, 1994–2003 (2000).
van de Wetering, M. et al. The human T cell transcription factor-1 gene. Structure, localization, and promoter characterization. J. Biol. Chem. 267, 8530–8536 (1992).
Van de Wetering, M., Castrop, J., Korinek, V. & Clevers, H. Extensive alternative splicing and dual promoter usage generate Tcf-protein isoforms with differential transcription control properties. Mol. Cell. Biol. 16, 745–752 (1996).
Morin, P. et al. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275, 1787–1790 (1997).
Kengaku, M. et al. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science 280, 1274–1277 (1998).
Prieve, M.G. & Waterman, M.L. Nuclear localization and formation of β-catenin-lymphoid enhancer factor-1 complexes are not sufficient to activate gene expression. Mol. Cell. Biol. 19, 4503–4515 (1999).
Aoki, M., Hecht, A., Kruse, U., Kemler, R. & Vogt, P.K. Nuclear endpoint of Wnt signaling: neoplastic transformation induced by transactivating lymphoid-enhancing factor 1. Proc. Natl. Acad. Sci. USA 96, 139–144 (1999).
Spolski, R. et al. Regulation of expression of T cell γ chain, L3T4 and Ly-2 messages in Abelson/Moloney virus-transformed T cell lines. Eur. J. Immunol. 18, 295–300 (1988).
Carlsson, P., Waterman, M. & Jones, K. The hLEF/TCF-1α HMG protein contains a context-dependent transcriptional activation domain that induces the TCRα enhancer in T cells. Genes Dev. 7, 2418–2430 (1993).
Acknowledgements
We thank H. Clevers for TCF1 and TCF4 expression plasmids and the TOPtk/FOPtk reporters; M. Schlissel for the 2017 cell line; J. Ta for assisting with the sequencing of genomic clones; J. Groden and K. Heppner Goss for the GFP/APC construct and advice; T. Pawloski for assistance with the image analysis of the in situ hybridization; and E. Stanbridge, A. Syed, O. Marcu, B. Semler, S. Sandmeyer, H. Fan, H. Mangalam and members of the Waterman and Marsh laboratories for suggestions and critique. This work was supported by the Chao Family Comprehensive Cancer Center Functional Genomics Program to J.L.M., R.F.H. and M.L.W, the UCI College of Medicine to R.F.H., NIH Research Grants RO1 HD36081 and RO1 HD36049 to J.L.M., and NIH R01 CA-83982 to M.L.W.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hovanes, K., Li, T., Munguia, J. et al. β-catenin–sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet 28, 53–57 (2001). https://doi.org/10.1038/ng0501-53
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0501-53
This article is cited by
-
Dickkopf1 induces enteric neurogenesis and gliogenesis in vitro if apoptosis is evaded
Communications Biology (2023)
-
PCBP1-mediated regulation of WNT signaling is critical for breast tumorigenesis
Cell Biology and Toxicology (2023)
-
Secreted Frizzled-Related Protein 2 (sFRP2) Functions as a Melanogenic Stimulator; the Role of sFRP2 in UV-Induced Hyperpigmentary Disorders
Journal of Investigative Dermatology (2016)
-
Ring Finger Protein 14 is a new regulator of TCF/β‐catenin‐mediated transcription and colon cancer cell survival
EMBO reports (2013)
-
Dual functions of DP1 promote biphasic Wnt-on and Wnt-off states during anteroposterior neural patterning
The EMBO Journal (2012)