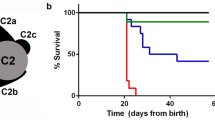
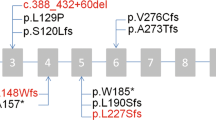
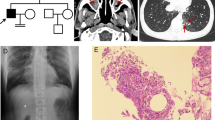
Abstract
Primary ciliary dyskinesia (PCD) is an inherited disorder characterized by recurrent infections of the upper and lower respiratory tract, reduced fertility in males and situs inversus in about 50% of affected individuals (Kartagener syndrome). It is caused by motility defects in the respiratory cilia that are responsible for airway clearance, the flagella that propel sperm cells and the nodal monocilia that determine left-right asymmetry1. Recessive mutations that cause PCD have been identified in genes encoding components of the outer dynein arms, radial spokes and cytoplasmic pre-assembly factors of axonemal dyneins, but these mutations account for only about 50% of cases of PCD. We exploited the unique properties of dog populations to positionally clone a new PCD gene, CCDC39. We found that loss-of-function mutations in the human ortholog underlie a substantial fraction of PCD cases with axonemal disorganization and abnormal ciliary beating. Functional analyses indicated that CCDC39 localizes to ciliary axonemes and is essential for assembly of inner dynein arms and the dynein regulatory complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Zariwala, M.A. et al. Genetic defects in ciliary structure and function. Annu. Rev. Physiol. 69, 423–450 (2007).
Cavrenne, R. et al. Primary ciliary dyskinesia and situs inversus in a young dog. Vet. Rec. 163, 54–55 (2008).
Randolph, J.F. & Castleman, W.L. Immotile cilia syndrome in two Old-English sheep dog littermates. J. Small Anim. Pract. 25, 679–686 (1984).
Gherman, A. et al. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat. Genet. 38, 961–962 (2006).
Inglis, P.N. et al. Piecing together a ciliome. Trends Genet. 22, 491–500 (2006).
Merchant, S.S. et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245–250 (2007).
Pazour, G.J. et al. Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 170, 103–113 (2005).
McClintock, T.S. et al. Tissue expression patterns identify mouse cilia genes. Physiol. Genomics 32, 198–206 (2008).
Omran, H. et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature 456, 611–616 (2008).
Baker, K. et al. Direct and indirect roles for Nodal signaling in two axis conversions during asymmetric morphogenesis of the zebrafish heart. Proc. Natl. Acad. Sci. USA 105, 13924–13929 (2008).
Papon, J.F. et al. A 20-year experience of electron microscopy in the diagnosis of primary ciliary dyskinesia. Eur. Respir. J. 35, 1057–1063 (2010).
Kennedy, M.P. et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation 115, 2814–2821 (2007).
Fliegauf, M. et al. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 171, 1343–1349 (2005).
Heuser, T. et al. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J. Cell Biol. 187, 921–933 (2009).
Colantonio, J.R. et al. The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. Nature 457, 205–209 (2009).
Hill, K.L. et al. T lymphocyte-triggering factor of African trypanosomes is associated with the flagellar fraction of the cytoskeleton and represents a new family of proteins that are present in several divergent eukaryotes. J. Biol. Chem. 275, 39369–39378 (2000).
Ralston, K.S. et al. Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system. Eukaryot. Cell 5, 696–711 (2006).
Rupp, G. & Porter, M.E. A subunit of the dynein regulatory complex in Chlamydomonas is a homologue of a growth arrest-specific gene product. J. Cell Biol. 162, 47–57 (2003).
Becker-Heck, A. et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat. Genet. published online, doi: 10.1038/ng.727 (5 December 2010).
Huang, B., Ramanis, Z. & Luck, D.J. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell 28, 115–124 (1982).
Piperno, G., Mead, K. & Shestak, W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J. Cell Biol. 118, 1455–1463 (1992).
Piperno, G., Mead, K., LeDizet, M. & Moscatelli, A. Mutations in the “dynein regulatory complex” alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J. Cell Biol. 125, 1109–1117 (1994).
Piperno, G., Mead, K. & Henderson, S. Inner dynein arms but not outer dynein arms require the activity of kinesin homologue protein KHP1 (FLA10) to reach the distal part of flagella in Chlamydomonas. J. Cell Biol. 133, 371–379 (1996).
Chang, B. et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum. Mol. Genet. 15, 1847–1857 (2006).
Charlier, C. et al. Highly effective SNP based association mapping and management of recessive defects in livestock. Nat. Genet. 40, 449–454 (2008).
Ge, B. et al. Survey of allelic expression using EST mining. Genome Res. 15, 1584–1591 (2005).
Olbrich, H. et al. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat. Genet. 30, 143–144 (2002).
Olbrich, H. et al. Axonemal localization of the dynein component DNAH5 is not altered in secondary ciliary dyskinesia. Pediatr. Res. 59, 418–422 (2006).
Sisson, J.H., Stoner, J.A., Ammons, B.A. & Wyatt, T.A. All-digital capture and whole-field analysis of ciliary beat frequency. J. Microsc. 211, 103–111 (2003).
Acknowledgements
This work was supported by grants from the European Union (LUPA IP) and from the Police Scientifique Fédérale de Belgique (GENFUNC PAI) (to M.G.), from the Legs Poix from the Chancellerie des Universités, the Assistance Publique-Hôpitaux de Paris (PHRC AOM06053, P060245) and the Agence Nationale pour la Recherche (ANR-05-MRAR-022-01) (to S.A.), the US National Institutes of Health (HD04260, DK072301 and DK075972 (to N.K.) and DK079541 (to E.E.D.)) and by grants from the “Deutsche Forschungsgemeinschaft” DFG Om 6/4, GRK1104, SFB592, and the European Community (EU-CILIA; SYS-CILIA) (to H.O.). A.C.M. is a fellow from the FRIA. N.K. is the Jean and George W. Brumley Professor. Y.M. benefits from a postdoctoral fellowship to study abroad from the Japanese Society for the Promotion of Science (JSPS). We thank the Bobtail breeders for assistance; patients and their family members whose cooperation made this study possible; all referring physicians; the German patient support group “Kartagener Syndrom und Primaere Ciliaere Dyskinesie e.V.”; K. Nakamura and the GIGA-R genomics platform for their contribution to sequencing; E. Ostrander for samples from healthy Old English Sheepdogs; the Unité de Recherche Clinique (URC) Est (AP-HP, Hôpital Saint-Antoine, Paris, France) for support; and A. Heer, C. Reinhard, C. Kopp, K. Sutter, M. Petry, C. Tessmer, A.-M. Vojtek and S. Franz for technical assistance.
Author information
Authors and Affiliations
Contributions
The positional cloning of CCDC39 in the dog was performed by A.-C.M. and G.B. Genome-wide SNP genotyping was conducted at CNG under supervision of M. Lathrop and D.Z. Mining the ciliome databases was conducted by E.E.D. Experiments in the zebrafish were conducted by E.E.D. In situ hybridization in the mouse was conducted by A.K. and H.O. qRT-PCR on human samples was conducted by M. Legendre, P.D., G.M. and H.T. Sequencing of CCDC39 in human subjects, including high-throughput sequencing, was conducted by A.-C.M., G.B., Y.M., A.B.-H., M. Legendre, E.E., P.D., G.M. and H.T. Identifying the p.Glu390SerfsX6 mutation was realized by M. Legendre, P.D., G.M. and H.T. Haplotype analysis to determine founder status of CCDC39 mutations was conducted by M. Legendre and P.D. TEM analysis was conducted by M.J. (dog), E.E. and D.E. (French cohort), and by K.G.N., J.K.M., H.O. and routine laboratories (German cohort). High-resolution immunofluorescence analyses were done by A.B.-H., M.F., J.H. and N.T.L. Immunoblotting analyses were done by A.B.-H. High-speed video analyses were conducted by H.O., N.T.L. and A.B.-H. Monoclonal anti-GAS11 antibody was produced by A.B.-H. and H.Z. Polyclonal anti-GAS11 antibodies were provided by K.H. and R.C. Clinical examination and collection of the canine PCD cases was conducted by F.B., C.C. and S.D. M.G., A.-S.L., N.K., H.O. and S.A. designed experiments, analyzed data and wrote the manuscript. All remaining authors as well as H.O., K.G.N. and J.K.M. examined and contributed samples from individuals with PCD or heterotaxia.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Figures 1–9 and Supplementary Table 1 (PDF 4139 kb)
Supplemental Video 1
Ciliary beating pattern of PCD patient OP122 with CCDC39 mutations assessed by high-speed videomicroscopy analyses of respiratory cells obtained by nasal brushing biopsy (AVI 1118 kb)
Supplemental Video 2
Ciliary beating pattern of a healthy control individual assessed by high-speed videomicroscopy analyses of respiratory cells obtained by nasal brushing biopsy (AVI 4582 kb)
Rights and permissions
About this article
Cite this article
Merveille, AC., Davis, E., Becker-Heck, A. et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat Genet 43, 72–78 (2011). https://doi.org/10.1038/ng.726
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.726
This article is cited by
-
Characterization of a DRC1 null variant associated with primary ciliary dyskinesia and female infertility
Journal of Assisted Reproduction and Genetics (2023)
-
Dynein axonemal heavy chain 10 deficiency causes primary ciliary dyskinesia in humans and mice
Frontiers of Medicine (2023)
-
The dynamics of protein localisation to restricted zones within Drosophila mechanosensory cilia
Scientific Reports (2022)
-
A change of heart: new roles for cilia in cardiac development and disease
Nature Reviews Cardiology (2022)
-
The regulatory roles of motile cilia in CSF circulation and hydrocephalus
Fluids and Barriers of the CNS (2021)