Abstract
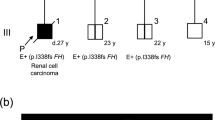
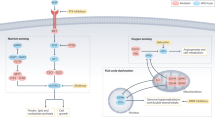
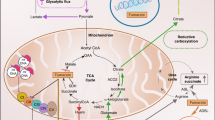
Renal cell carcinoma (RCC) represents a group of diseases linked by their primary site of origin, the kidney. Studies of families with a genetic predisposition to the development of kidney cancer have revealed that multiple genes are involved in the molecular pathogenesis of RCC. Germline mutations in a gene that encodes a Krebs cycle enzyme have been found to result in a distinct clinical entity referred to as hereditary leiomyomatosis and renal cell cancer (HLRCC). HLRCC is inherited in an autosomal-dominant fashion. Affected individuals in HLRCC families are at risk for the development of leiomyomas of the skin and uterus as well as renal cancers. HLRCC-associated kidney tumors are often biologically aggressive. Linkage analysis has identified germline alterations in the fumarate hydratase (FH) gene associated with HLRCC. While the mechanisms of molecular carcinogenesis are not entirely understood, several lines of evidence derived from clinical and basic research suggest that pseudohypoxia might drive cellular transformation. The role of FH mutations in sporadic tumors seems to be limited. Nevertheless, continued investigation of HLRCC should provide further insight into the mechanisms of kidney cancer development, and could potentially identify targets for new therapeutic approaches to RCC.
Key Points
-
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is a genetic disease that predisposes individuals to the development of leiomyomas of the skin and uterus as well as kidney cancer
-
Kidney cancers in patients with HLRCC are often biologically aggressive
-
HLRCC is caused by germline mutations of the FH gene, which encodes the Krebs cycle enzyme fumarate hydratase
-
Fumarate-induced pseudohypoxia could drive tumor formation
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Launonen V et al. (2001) Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci USA 98: 3387–3392
Tomlinson IP et al. (2002) Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 30: 406–410
Wei MH et al. (2006) Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet 43: 18–27
Toro JR et al. (2003) Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet 73: 95–106
Kiuru M et al. (2002) Few FH mutations in sporadic counterparts of tumor types observed in hereditary leiomyomatosis and renal cell cancer families. Cancer Res 62: 4554–4557
Reed WB et al. (1973) Cutaneous leiomyomata with uterine leiomyomata. Acta Derm Venereol 53: 409–416
Alam NA et al. (2003) Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet 12: 1241–1252
Merino MJ et al. (2003) Hereditary leiomyomatosis and renal cell carcinoma syndrome (HLRCC); clinical, histopathological and molecular features of the first American families described [abstract]. Mod Pathol 16: 739
Knudson AG Jr (1971) Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 68: 820–823
Gellera C et al. (1990) Fumarase deficiency is an autosomal recessive encephalopathy affecting both the mitochondrial and the cytosolic enzymes. Neurology 40: 495–499
Friedrich CA (2001) Genotype–phenotype correlation in von Hippel–Lindau syndrome. Hum Mol Genet 10: 763–767
Gnarra JR et al. (1994) Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 7: 85–90
Shuin T et al. (1994) Frequent somatic mutations and loss of heterozygosity of the von Hippel–Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res 54: 2852–2855
Morris MR et al. (2004) Molecular genetic analysis of FIH-1, FH, and SDHB candidate tumour suppressor genes in renal cell carcinoma. J Clin Pathol 57: 706–711
Astuti D et al. (2001) Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 69: 49–54
Niemann S and Muller U (2000) Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet 26: 268–270
Baysal BE et al. (2000) Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287: 848–851
Vanharanta S et al. (2004) Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet 74: 153–159
Gottlieb E and Tomlinson IP (2005) Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer 5: 857–866
Pause A et al. (1997) The von Hippel–Lindau tumor-suppressor gene product forms a stable complex with human Cul-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci USA 94: 2156–2161
Kaelin WG Jr (2002) Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2: 673–682
Kaelin WG Jr (2003) The von Hippel–Lindau gene, kidney cancer, and oxygen sensing. J Am Soc Nephrol 14: 2703–2711
Pugh CW and Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9: 677–684
Linehan WM et al. (2003) The genetic basis of cancer of the kidney. J Urol 170: 2163–2172
Bruick RK and McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294: 1337–1340
Epstein AC et al. (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54
Ivan M et al. (2001) HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468
Jaakkola P et al. (2001) Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472
Yu F et al. (2001) HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA 98: 9630–9635
Isaacs JS et al. (2005) HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8: 143–153
Iliopoulos O et al. (1996) Negative regulation of hypoxia-inducible genes by the von Hippel–Lindau protein. Proc Natl Acad Sci USA 93: 10595–10599
Wiesener MS et al. (2001) Constitutive activation of hypoxia-inducible genes related to overexpression of hypoxia-inducible factor-1α in clear cell renal carcinomas. Cancer Res 61: 5215–5222
Pollard PJ et al. (2005) Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum Mol Genet 14: 2231–2239
Pollard P et al. (2005) Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol 205: 41–49
Selak MA et al. (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7: 77–85
Edwards YH and Hopkinson DA (1979) The genetic determination of fumarase isozymes in human tissues. Ann Hum Genet 42: 303–313
Linehan WM et al. (2004) Genetic basis of cancer of the kidney: disease-specific approaches to therapy. Clin Cancer Res 10 (Pt 2): 6282S–6289S
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sudarshan, S., Pinto, P., Neckers, L. et al. Mechanisms of Disease: hereditary leiomyomatosis and renal cell cancer—a distinct form of hereditary kidney cancer. Nat Rev Urol 4, 104–110 (2007). https://doi.org/10.1038/ncpuro0711
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro0711
This article is cited by
-
Primary bilateral macronodular adrenal hyperplasia: definitely a genetic disease
Nature Reviews Endocrinology (2022)
-
Direct and quantitative analysis of altered metabolic flux distributions and cellular ATP production pathway in fumarate hydratase-diminished cells
Scientific Reports (2020)
-
Renal cell carcinoma: translational aspects of metabolism and therapeutic consequences
Kidney International (2013)
-
Leiomyoma: genetics, assisted reproduction, pregnancy and therapeutic advances
Journal of Assisted Reproduction and Genetics (2012)
-
Exploring a glycolytic inhibitor for the treatment of an FH-deficient type-2 papillary RCC
Nature Reviews Urology (2011)