Abstract
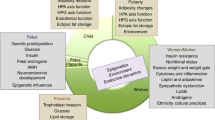
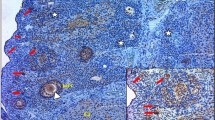
The symptoms of women with polycystic ovary syndrome (PCOS) include hirsutism and irregular menstrual bleeding due to ovarian androgen excess and chronic anovulation. Typically, these features emerge late in puberty or shortly thereafter. The proposed mechanism(s) responsible for increased ovarian androgen production include heightened theca cell responsiveness to gonadotropin stimulation, increased pituitary secretion of luteinizing hormone, and hyperinsulinemia. The cause of ovulatory dysfunction is not well understood, but is linked to abnormal follicle growth and development within the ovary. As a result, infertility is common among women with PCOS and, in many instances, is the initial presenting complaint. Insulin resistance and obesity are frequently associated with PCOS and probably contribute to the severity of symptoms. The polycystic ovary that accompanies the syndrome has recently been defined as having 12 or more follicles per ovary or an ovarian volume greater than 10 ml as determined by ultrasonography. In addition, there is an increased number of growing follicles in the polycystic ovary. Despite this distinctive appearance, the cause and development of the polycystic ovary are completely unknown.
Key Points
-
Diagnostic criteria for polycystic ovary syndrome (PCOS) include hirsutism, irregular menstrual bleeding and evidence of polycystic ovaries
-
In women with PCOS, the primary source of excess androgen production is the ovary
-
The mechanism for irregular menstrual bleeding is endometrial responses to persistent and variable unopposed estrogen secretion
-
Women with PCOS at increased risk for endometrial adenocarcinoma
-
The presence of obesity and insulin resistance increases the severity of clinical presentation in women with PCOS
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Azziz R et al. (2004) Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab 89: 453–462
Conway GS et al. (1989) Heterogeneity of the polycystic ovary syndrome: clinical, endocrine and ultrasound features in 556 patients. Clin Endocrinol 30: 459–470
Legro RS et al. (2004) Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv 59: 141–154
Carmina E et al. (1992) Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol 167: 1807–1812
Carmina E et al. (2003) Differences in body weight between American and Italian women with polycystic ovary syndrome: influence of the diet. Hum Reprod 18: 2289–2293
The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81: 19–25
The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19: 41–47
Erickson GF (1992) Folliculogenesis in polycystic ovary syndrome. In Current Issues in Endocrinology and Metabolism: Polycystic Ovary Syndrome, 111–128 (Eds Dunaif A et al.) Cambridge, MA: Blackwell Scientific Publishers
Hughesdon P (1982) Morphology and morphogenesis of the stein-leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv 37: 59–77
Maciel GA et al. (2004) Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab 89: 5321–5327
Webber LJ et al. (2003) Formation and early development of follicles in the polycystic ovary. Lancet 362: 1017–1021
Weenen C et al. (2004) Anti-mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10: 77–83
Stubbs SA et al. (2005) Anti-mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab 90: 5536–5543
Wachs DS et al. (2007) Serum anti-mullerian hormone concentrations are not affected by acute administration of follicle stimulating hormone in polycystic ovary syndrome and normal women. J Clin Endocrinol Metab 92: 1871–1874
Erickson GF et al. (1989) Theca function in polycystic ovaries of a patient with virilizing congenital adrenal hyperplasia. Fertil Steril 51: 173–176
Dunaif A et al. (1984) The effects of continuous androgen secretion on the hypothalamic-pituitary axis in women: evidence from a luteinized thecoma of the ovary. J Clin Endocrinol Metab 59: 389–393
Barnes RB et al. (1994) Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab 79: 1328–1333
Futterweit W and Deligdisch L (1986) Histopathological effects of exogenously administered testosterone in 19 female to male transsexuals. J Clin Endocrinol Metab 62: 16–21
Spinder T et al. (1989) The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J Clin Endocrinol Metab 69: 151–157
Chadha S et al. (1994) Androgen receptor expression in human ovarian and uterine tissue of long term androgen-treated transsexual women. Hum Pathol 25: 1198–1204
Vendola KA et al. (1998) Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest 101: 2622–2629
Nelson VL et al. (1999) Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol 13: 946–957
Wickenheisser JK et al. (2006) Human ovarian theca cells in culture. Trends Endocrinol Metab 17: 65–71
Barnes RB et al. (1989) Pituitary-ovarian responses to nafarelin testing in the polycystic ovary syndrome. N Engl J Med 320: 559–565
Ehrmann DA et al. (1995) Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev 16: 322–353
Gilling-Smith C et al. (1997) Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol 47: 93–99
Lobo RA et al. (1983) Elevated bioactive luteinizing hormone in women with the polycystic ovary syndrome. Fertil Steril 39: 674–678
Chang R et al. (1983) Steroid secretion in polycystic ovarian disease after ovarian suppression by a long-acting gonadotropin-releasing hormone agonist. J Clin Endocrinol Metab 56: 897–903
El-Roeiy A et al. (1994) Expression of the genes encoding the insulin-like growth factors IGF-I and II, the IGF and insulin receptors, and IGF-binding proteins-1-6 and the localization of their gene products in normal and polycystic ovary syndrome ovaries. J Clin Endocrinol Metab 78: 1488–1496
Willis D and Franks S (1995) Insulin action in human granulosa cells from normal and polycystic ovaries is mediated by the insulin receptor and not the type-I insulin-like growth factor receptor. J Clin Endocrinol Metab 80: 3788–3790
Bergh C et al. (1993) Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril 59: 323–331
Barbieri RL et al. (1984) Insulin stimulates androgen accumulation in incubations of human ovarian stroma and theca. Obstet Gynecol 64: 73S–80S
Barbieri RL et al. (1986) Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab 62: 904–910
Nestler JE et al. (1998) Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 83: 2001–2005
Nestler JE et al. (1987) The effects of hyperandrogenemia on serum testosterone, progesterone, dehydroepiandrosterone sulfate, and cortisol levels in normal women and in a woman with hyperandrogenism, insulin resistance, and acanthosis nigricans. J Clin Endocrinol Metab 64: 180–184
Dunaif A and Graf M (1989) Insulin administration alters gonadal steroid metabolism independent of changes in gonadotropin secretion in insulin-resistant women with polycystic ovary syndrome J Clin Invest 83: 23–29
Plymate SR et al. (1988) Inhibition of sex hormone-binding globulin production in the human hepatoma (hep g2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67: 460–464
Crave JC et al. (1995) Differential effects of insulin and insulin-like growth factor I on the production on plasma steroid binding globulins by human hepatoblastoma-derived (hepg2) cells. J Clin Endocrinol Metab 80: 1283–1289
Lassmann-Vague V et al. (1994) SHBG (sex hormone-binding globulin) levels in insulin dependent diabetic patients according to the route of insulin administration. Horm Metab Res 26: 436–437
Ducluzeau P-H et al. (2003) Glucose-to-insulin ratio rather than sex hormone-binding globulin and adiponectin levels is the best predictor of insulin resistance in nonobese women with polycystic ovary syndrome. J Clin Endocrinol Metab 88: 3626–3631
Mingrone G et al. (2002) Sex hormone-binding globulin levels and cardiovascular risk factors in morbidly obese subjects before and after weight reduction induced by diet or malabsorptive surgery. Atherosclerosis 161: 455–462
Escobar-Morreale HF et al. (2005) The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev 26: 251–282
Erickson GF et al. (1990) The effects of insulin and insulin-like growth factors-I and -II on estradiol production by granulosa cells of polycystic ovaries. J Clin Endocrinol Metab 70: 894–902
Coffler MS et al. (2003) Enhanced granulosa cell responsiveness to follicle stimulating hormone during insulin infusion in women with polycystic ovary syndrome treated with pioglitazone. J Clin Endocrinol Metab 88: 5624–5631
Almahbobi G et al. (1996) Functional integrity of granulosa cells from polycystic ovaries. Clin Endocrinol 44: 571–580
Nimrod A (1977) Studies on the synergistic effect of androgen on the stimulation of progestin secretion by FSH in cultured rat granulosa cells: progesterone metabolism and the effect of androgens. Mol Cell Endocrinol 8: 189–199
Daniel S and Armstrong D (1980) Enhancement of follicle-stimulating hormone-induced aromatase activity by androgens in cultured rat granulosa cells. Endocrinology 107: 1027–1033
Hillier S and De Zwart F (1981) Evidence that granulosa cell aromatase induction/activation by follicle-stimulating hormone is an androgen receptor-regulated process in-vitro. Endocrinology 109: 1303–1305
Harlow C et al. (1986) Androgen modulation of follicle-stimulating hormone-induced granulosa cell steroidogenesis in the primate ovary. Endocrinology 119: 1403–1405
Daniel SAJ and Armstrong DT (1984) Site of action of androgens on follicle-stimulating hormone-induced aromatase activity in cultured rat granulosa cells. Endocrinology 114: 1975–1982
Weil SJ et al. (1998) Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab 83: 2479–2485
Willis D et al. (1996) Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab 81: 302–309
Velazquez E et al. (1997) Menstrual cyclicity after metformin therapy in polycystic ovary syndrome. Obstet Gynecol 90: 392–395
Azziz R et al. (2001) Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab 86: 1626–1632
DeFazio J et al. (1985) Acute ovarian responses to a long-acting agonist of gonadotropin-releasing hormone in ovulatory women and women with polycystic ovarian disease. Fertil Steril 44: 453–459
Van Der Meer M et al. (1998) Cohort size rather than follicle-stimulating hormone threshold level determines ovarian sensitivity in polycystic ovary syndrome. J Clin Endocrinol Metab 83: 423–426
Rebar R et al. (1976) Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest 57: 1320–1329
Kaiser U et al. (1995) A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Physiology 92: 12280–12284
Hoff J et al. (1979) The functional relationship between priming and releasing actions of luteinizing hormone-releasing hormone. J Clin Endocrinol Metab 49: 8–11
Hall J et al. (1998) Insights into hypothalamic-pituitary dysfunction in polycystic ovary syndrome. J Endocrinol Invest 21: 602–611
Pastor CL et al. (1998) Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 83: 582–590
Eagleson CA et al. (2000) Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 85: 4047–4052
Adashi EY et al. (1981) Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology 108: 1441–1449
Dunaif A and Graf M (1989) Insulin administration alters gonadal steroid metabolism independent of changes in gonadotropin secretion in insulin-resistant women with the polycystic ovary syndrome. J Clin Invest 83: 23–29
Mehta RV et al. (2005) Luteinizing hormone secretion is not influenced by insulin infusion in women with polycystic ovary syndrome despite improved insulin sensitivity during pioglitazone treatment. J Clin Endocrinol Metab 90: 2136–2141
Acknowledgements
This work was supported in part by the National Institute of Child Health and Human Development (NICHD) and NIH through cooperative agreement U54 HD 12303-20 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and in part by NIH grant MO1 RR00827. Désirée Lie, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Chang, R. The reproductive phenotype in polycystic ovary syndrome. Nat Rev Endocrinol 3, 688–695 (2007). https://doi.org/10.1038/ncpendmet0637
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0637
This article is cited by
-
Molecular Mechanisms in the Etiology of Polycystic Ovary Syndrome (PCOS): A Multifaceted Hypothesis Towards the Disease with Potential Therapeutics
Indian Journal of Clinical Biochemistry (2024)
-
Effects of exogenous adiponectin supplementation in early pregnant PCOS mice on the metabolic syndrome of adult female offspring
Journal of Ovarian Research (2021)
-
Associated Effects of Endocrine Disrupting Chemicals (EDCs) on Neuroendocrine Axes and Neurotransmitter Profile in Polycystic Ovarian Syndrome Condition
Proceedings of the Zoological Society (2021)
-
Potential of Auraptene in Improvement of Oocyte Maturation, Fertilization Rate, and Inflammation in Polycystic Ovary Syndrome Mouse Model
Reproductive Sciences (2020)
-
Weight management merits attention in women with infertility: a cross-sectional study on the association of anthropometric indices with hormonal imbalance in a Ghanaian population
BMC Research Notes (2019)