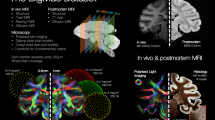
Abstract
A major goal of modern MRI research is to be able to image neural circuits in the central nervous system. Critical to this mission is the ability to describe a number of important parameters associated with neural circuits. These parameters include neural architecture, functional activation of neural circuits, anatomical and functional connectivity of neural circuits, and factors that might alter neural circuits, such as trafficking of immune cells and brain precursor cells in the brain. Remarkably, a variety of work in human and animal brains has demonstrated that all these features of neural circuits can be visualized with MRI. In this Article we provide a brief summary of the new directions in neural imaging research, which should prove useful in future analyses of normal and pathological human brains and in studies of animal models of neurological and psychiatric disorders. At present, few MRI data characterizing the neural circuits in the heart are available, but in this Article we discuss the applicable present developments and the prospects for the future.
Key Points
-
MRI can be sensitized to measure various aspects of neural circuits through the use of endogenous and exogenous contrast agents
-
Intrinsic magnetic-susceptibility contrast in human brain or manganese injection in animal brain can be used to reveal the laminar subdivisions of the cerebral cortex
-
Contrast agents that make use of the processes of water diffusion and of manganese transport allow detection of major fiber tracts
-
MRI-based detection of blood flow fluctuations that result from neuronal activity allows identification of the functional subdivisions of the brain
-
Novel contrast agents allow tracking of stem cells and of infiltration of a variety of organs by immune cells
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Essig M et al. (2006) Contrast-enhanced magnetic resonance imaging of central nervous system tumors: agents, mechanisms, and applications. Top Magn Reson Imaging 17: 89–106
Rovaris M and Filippi M (2000) Contrast enhancement and the acute lesion in multiple sclerosis. Neuroimaging Clin N Am 10: 705–116
Marcu CB et al. (2007) Delayed contrast enhancement magnetic resonance imaging for the assessment of cardiac disease. Heart Lung Circ 16: 70–78
Le Bihan D (2003) Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4: 469–480
Heiss WD and Sorensen AG (2007) Advances in imaging 2006. Stroke 38: 238–240
Mori S and Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51: 527–539
Sundgren PC et al. (2004) Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology 46: 339–550
Tseng WY et al. (2003) Diffusion tensor MRI of myocardial fibers and sheets: correspondence with visible cut-face texture. J Magn Reson Imaging 17: 31–42
Belliveau JW et al. (1991) Functional studies of the human brain using high-speed magnetic resonance imaging. J Neuroimaging 1: 36–41
Kwong KK et al. (1992) Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 89: 5675–5679
Ogawa S et al. (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87: 9868–9872
D'Esposito M (2000) Functional neuroimaging of cognition. Semin Neurol 20: 487–498
Cabeza R and Nyberg L (2000) Imaging cognition, II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47
Hsu LY et al. (2006) Quantitative myocardial perfusion analysis with a dual-bolus contrast-enhanced first-pass MRI technique in humans. J Magn Reson Imaging 23: 315–322
Brodmann K (1909) Vergleichende Lokalisationlehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues [German]. Leipzig: Barth-Verlag
Vogt O (1911) Die myeloarchitektonik des isocortex parietalis [German]. J Psychol Neurol 18: 107–118
Annese J et al. (2004) A myelo-architectonic method for the structural classification of cortical areas. Neuroimage 21: 15–26
Clark VP et al. (1992) In vivo myeloarchitectonic analysis of human striate and extrastriate cortex using magnetic resonance imaging. Cereb Cortex 2: 417–424
Barbier EL et al. (2002) Imaging cortical anatomy by high-resolution MR at 3.0T: detection of the stripe of Gennari in visual area 17. Magn Reson Med 48: 735–738
Bridge H and Clare S (2006) High-resolution MRI: in vivo histology. Philos Trans R Soc Lond B Biol Sci 361: 137–146
Walters NB et al. (2003) In vivo identification of human cortical areas using high-resolution MRI: an approach to cerebral structure-function correlation. Proc Natl Acad Sci USA 100: 2981–2986
Xu F et al. (2003) Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci USA 100: 11029–11034
Watanabe T et al. (2002) In vivo 3D MRI staining of mouse brain after subcutaneous application of MnCl2. Magn Reson Med 48: 852–859
Aoki I et al. (2004) In vivo detection of neuroarchitecture in the rodent brain using manganese-enhanced MRI. Neuroimage 22: 1046–1059
Alvestad S et al. (2007) In vivo mapping of temporospatial changes in manganese enhancement in rat brain during epileptogenesis. Neuroimage 38: 57–66
Wendland MF (2004) Applications of manganese-enhanced magnetic resonance imaging (MEMRI) to imaging of the heart. NMR Biomed 17: 581–594
Duyn JH et al. (2007) High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci USA 104: 11796–11801
de Zwart JA et al. (2004) Signal-to-noise ratio and parallel imaging performance of a 16-channel receive-only brain coil array at 3.0 Tesla. Magn Reson Med 51: 22–26
Wiggins GC et al. (2006) 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med 56: 216–223
Li TQ et al. (2006) Extensive heterogeneity in white matter intensity in high-resolution T2*-weighted MRI of the human brain at 7.0 T. Neuroimage 32: 1032–1040
Reichenbach JR et al. (1997) Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 204: 272–277
Craelius W et al. (1982) Iron deposits surrounding multiple sclerosis plaques. Arch Pathol Lab Med 106: 397–399
Benveniste H et al. (1999) Detection of neuritic plaques in Alzheimer's disease by magnetic resonance microscopy. Proc Natl Acad Sci USA 96: 14079–14084
Jack CR Jr et al. (2005) In vivo magnetic resonance microimaging of individual amyloid plaques in Alzheimer's transgenic mice. J Neurosci 25: 10041–10048
Zhang J et al. (2004) Detection of amyloid plaques in mouse models of Alzheimer's disease by magnetic resonance imaging. Magn Reson Med 51: 452–457
Sehgal V et al. (2005) Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging 22: 439–450
Wycliffe ND et al. (2004) Reliability in detection of hemorrhage in acute stroke by a new three-dimensional gradient recalled echo susceptibility-weighted imaging technique compared to computed tomography: a retrospective study. J Magn Reson Imaging 20: 372–377
Vignaud A et al. (2006) Detection of myocardial capillary orientation with intravascular iron-oxide nanoparticles in spin-echo MRI. Magn Reson Med 55: 725–730
Köhler S et al. (2003) Investigation of the microstructure of the isolated rat heart: a comparison between T*2- and diffusion-weighted MRI. Magn Reson Med 50: 1144–1150
Stephan KE et al. (2007) Dynamic causal models of neural system dynamics: current state and future extensions. J Biosci 32: 129–144
Ritter P and Villringer A (2006) Simultaneous EEG-fMRI. Neurosci Biobehav Rev 30: 823–838
Wong SW et al. (2007) Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage 35: 698–708
Colivicchi F et al. (2004) Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke 35: 2094–2098
Biswal B et al. (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541
Wise RG et al. (2004) Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage 21: 1652–1664
Fukunaga M et al. (2008) Metabolic origin of BOLD signal fluctuations in absence of stimuli. J Cereb Blood Flow Metab in press
Damoiseaux JS et al. (2006) Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853
Greicius MD et al. (2004) Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637–4642
Lowe MJ et al. (2002) Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology 224: 184–192
Iadecola C et al. (1997) Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol 78: 651–659
Lin YJ and Koretsky AP (1997) Manganese ion enhances T1-weighted MRI during brain activation: an approach to direct imaging of brain function. Magn Reson Med 38: 378–388
Yu X et al. (2005) In vivo auditory brain mapping in mice with Mn-enhanced MRI. Nat Neurosci 8: 961–968
Kuo YT et al. (2006) Manganese-enhanced magnetic resonance imaging (MEMRI) without compromise of the blood-brain barrier detects hypothalamic neuronal activity in vivo. NMR Biomed 19: 1028–1034
Hu TC et al. (2001) Manganese-enhanced MRI of mouse heart during changes in inotropy. Magn Reson Med 46: 884–890
Li WH et al. (2002) Mechanistic studies of a calcium-dependent MRI contrast agent. Inorg Chem 41: 4018–4024
Atanasijevic T et al. (2006) Calcium-sensitive MRI contrast agents based on superparamagnetic iron oxide nanoparticles and calmodulin. Proc Natl Acad Sci USA 103: 14707–14712
Bandettini PA et al. (2005) Direct detection of neuronal activity with MRI: fantasy, possibility, or reality? Appl Magn Reson 28: 1–30
Blagoev KB et al. (2007) Modelling the magnetic signature of neuronal tissue. Neuroimage 37: 137–148
Pautler RG et al. (1998) In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. Magn Reson Med 40: 740–748
Pautler RG (2006) Biological applications of manganese-enhanced magnetic resonance imaging. Methods Mol Med 124: 365–386
Silva AC et al. (2008) Detection of cortical laminar architecture using manganese enhanced MRI. J Neurosci Methods 167: 246–257
Smith KD et al. (2007) In vivo axonal transport rates decrease in a mouse model of Alzheimer's disease. Neuroimage 35: 1401–1408
van der Zijden JP et al. (2007) Changes in neuronal connectivity after stroke in rats as studied by serial manganese-enhanced MRI. Neuroimage 34: 1650–1657
Bilgen M et al. (2005) Manganese-enhanced MRI of rat spinal cord injury. Magn Reson Imaging 23: 829–832
Bulte JW et al. (2002) In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab 22: 899–907
Petry KG et al. (2007) Magnetic resonance imaging of human brain macrophage infiltration. Neurotherapeutics 4: 434–442
Politi LS (2007) MR-based imaging of neural stem cells. Neuroradiology 49: 523–534
Callera F and de Melo CM (2007) Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells' migration into the injured site. Stem Cells Dev 16: 461–466
Shapiro EM et al. (2006) In vivo detection of single cells by MRI. Magn Reson Med 55: 242–249
Shapiro EM et al. (2007) Antibody-mediated cell labeling of peripheral T cells with micron-sized iron oxide particles (MPIOs) allows single cell detection by MRI. Contrast Media Mol Imaging 2: 147–153
Shapiro EM et al. (2006) Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. Neuroimage 32: 1150–1157
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Duyn, J., Koretsky, A. Magnetic resonance imaging of neural circuits. Nat Rev Cardiol 5 (Suppl 2), S71–S78 (2008). https://doi.org/10.1038/ncpcardio1248
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio1248